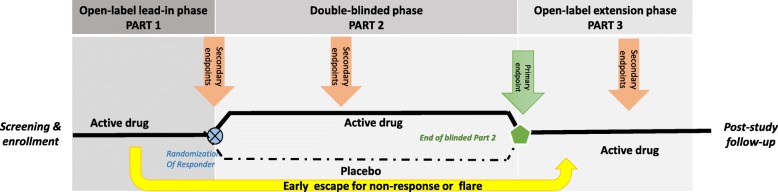
Fig. 1.
Basic design of Randomized Withdrawal Design. A randomized withdrawal design (RWD) study consists of three parts. During Part 1 and Part 3 all study participants receive open-label active study drug. Participants who show a clinically meaningful response to study drug by the end of Part 1 are randomized to the double-blinded placebo-controlled Part 2. Participants move from Part 2 to Part 3 if there is a flare event during Part 2 or upon completion of all Part 2 visits, whichever comes first. Patients for whom study drug may be beneficial, but who did not meet criteria for a meaningful improvement during Part 1, may be allowed to enter Part 3. The primary endpoint of a RWD trial is ‘the time to disease flare or the occurrence of a flare event during Part 2. The participant’s disease status at the end of Part 1 is used as the baseline to assess whether disease worsening (flare) has occurred during Part 2. Secondary RWD study endpoints can be measured throughout the duration of the entire RWD trial (Part 1 through 3) and include achievement of inactive disease, success in tapering certain background medications and change in patient reported outcomes