Abstract
Neurokinin-1 (NK-1) receptors are present in both the central nervous system and peripheral tissues. Substance P (SP) is the major ligand and is involved in multiple processes including pain transmission, vasodilation, modulation of the inflammatory response, as well as the sensory neuronal transmission involved in stress, anxiety, and emesis. The involvement of NK-1 and SP in the vomiting reflex has led to the development of NK-1 antagonists to prevent and treat vomiting in human and veterinary medicine. Maropitant is a potent, selective neurokinin (NK-1) receptor antagonist that blocks the pharmacologic action of SP in the central nervous system. Maropitant is available in both an injectable and tablet formulation and approved for use in dogs and cats for the treatment and prevention of vomiting from a variety of clinical causes and motion sickness. When administered prior to anesthetic premedication, maropitant prevents or significantly decreases the incidence of opioid-induced vomiting and signs of nausea in dogs and cats. Maropitant has also been shown to improve postoperative return to feeding and food intake in dogs. The minimum alveolar concentration of sevoflurage is decreased in both dogs and cats by maropitant, indicating a potential role as an adjunct analgesic, especially for visceral pain. This article will review the background information and literature, including clinical recommendations with respect to the perioperative use of maropitant in canine and feline veterinary patients.
Keywords: maropitant, perioperative nausea and vomiting, neurokinin-1 antagonist, Substance P
Introduction
Vomiting and nausea associated with anesthesia is a common occurrence in human medicine with an incidence of up to 80% in high-risk patients. The impact on human health care costs and patient distress is reflected in the recent publication where a systematic review of the literature from the years 2007–2011 identified over 2,600 articles pertaining to this issue.1 In veterinary medicine, the issue has only recently garnered attention. This may reflect an increasing focus on pain management and the use of mu-agonist opioid drugs for the treatment of moderate to severe pain. Morphine and hydromorphone are commonly used mu-agonist opioids in veterinary patients. Hydromorphone is a semi-synthetic derivative of morphine which is more lipid soluble and 4–8 times more potent than morphine. Unlike morphine, hydromorphone does not increase plasma histamine concentrations after intravenous (IV) administration. The incidence of vomiting in dogs has been documented as 50%–75% for morphine and 44%–100% for hydromorphone.2,3 The incidence of vomiting associated with opioids is affected by the specific drug and its lipid solubility profile, the dose and route of administration and the concomitant administration of other drugs such as acepromazine. In general, the incidence of vomiting is decreased with higher opioid doses, higher lipid solubility, and the prior administration of acepromazine, a dopamine antagonist.2,3,14
Multiple published studies have implicated perioperative vomiting as a risk factor for postoperative aspiration pneumonia in canine patients. Kogan et al4 found that in addition to esophageal, laryngeal, and neurological disorders, aspiration pneumonia was associated with vomiting and anesthesia and had a mortality rate of 33%. Alwood et al5 found that 22% of dogs undergoing laparotomy developed postoperative pulmonary complications. Of these cases, 75% were observed to have perioperative vomiting or regurgitation (50% pre-op, 4% intra-op, 21% post-op) with a 12% mortality. Tart et al6 identified potential risks associated with aspiration pneumonia and found that ~46% had recent general anesthesia or sedation and 64% were observed to have vomited perioperatively, resulting in 20% mortality. A recent multicenter study examined the prevalence and risk factors for canine postanesthetic aspiration pneumonia. The anesthesia factors associated with aspiration pneumonia included hydromorphone, administration of intraoperative constant-rate infusions, use of positive inotropes, and vomiting and regurgitation during or after anesthesia.7 In addition to decreasing the risk of postanesthetic aspiration pneumonia, preventing perioperative vomiting may also decrease morbidity in certain specific veterinary patient populations. Patients such as those with penetrating eye injuries, glaucoma, head trauma, or intracranial disease are specific examples where an increase in intraocular or intracranial pressure associated with vomiting should be avoided.
In addition to decreasing morbidity and mortality, preventing or treating perioperative vomiting and signs of nausea may also be considered an animal welfare issue. Human anesthesia patients report high levels of discomfort, distress, and dissatisfaction associated with perioperative nausea and vomiting. In fact, nausea and vomiting are the leading adverse events reported by human patients and are strongly related to patient dissatisfaction.9. Along with pain, nausea and vomiting are also ranked by human anesthesiologists as the top anesthesia outcomes that occur frequently and are important to avoid.8 Nausea is considered a prodromal sign of vomiting that may or may not result in vomiting. It is a highly subjective experience, and so clinical signs may be easily overlooked in veterinary patients who cannot self-report their discomfort. According to Brambell’s Five Freedoms of animal welfare, freedom from discomfort, pain, and distress should be worthy goals for veterinary practitioners. Interpretation of animal suffering dictates that if a human is likely to experience discomfort or distress under particular conditions, then it should be assumed that an animal of another species may be similarly affected.10
The importance of this issue to pet owners is reflected in a recent survey of dog owners presenting to a US veterinary teaching hospital. Of the 104 owners surveyed, an overwhelming number (over 90%) had at least some worry regarding their dog vomiting in relation to opioid analgesics and anesthesia and 46% indicated that they were moderately worried or very worried. Ninety-three percent expressed at least some concern about their dog experiencing nausea and 37.5% were moderately or very worried. When asked about treatment, 99% would probably or definitely choose treatment to prevent vomiting and close to 96% would probably or definitely choose treatment to decrease or prevent nausea. Furthermore, owners were willing to pay as much as $50–$75 (median and mean, respectively) for treatment, especially if it was recommended by their veterinarian. Most owners (>90%) were still likely or very likely to choose treatment even if they were required to arrive 1 hour earlier for their scheduled appointment in order to receive the treatment.11
An effective, US Food and Drug Administration-approved drug is available to prevent perioperative nausea and vomiting. Maropitant is a potent, selective neurokinin-1 (NK-1) antagonist which blocks the binding of the neurotransmitter Substance P (SP). SP is found in high concentrations in both the chemoreceptor trigger zone (CTZ) and the vomiting center (VC) and is a key neurotransmitter involved in vomiting. NK-1 antagonists work at both the CTZ and VC and, therefore, provide broad-spectrum inhibition of emesis. The remainder of this article will summarize published articles regarding the perianesthetic use of maropitant in dogs and cats and conclude with clinical recommendations based on these studies.
Background information
Tachykinins are a highly conserved group of peptides in mammalian species and are involved in many bodily processes, including neurotransmission and inflammation. The three primary tachykinins are SP, neurokinin A, and neurokinin B, and the three types of tachykinin receptors are based on their ligands.12 The NK-1 receptor has preferential affinity for SP, whereas NK-2 binds neurokinin A and NK-3 binds neurokinin B.12 NK-1 receptors are found in both the central nervous system and peripheral tissues and are involved in pain transmission, vasodilation, modulation of the inflammatory response, as well as the sensory neuronal transmission involved in stress, anxiety, and emesis.12 The ubiquitous nature of SP and NK-1 receptors in many biological functions including upregulation in pathological conditions makes it an important target for pain and vomiting and potentially depression, migraine, addiction, neuronal degeneration, and infection in human medicine.12 In human medicine, clinical development has focused on the drug aprepitant and its intravenous prodrug fosaprepitant for the treatment of anesthesia and chemotherapy-induced nausea and vomiting.12
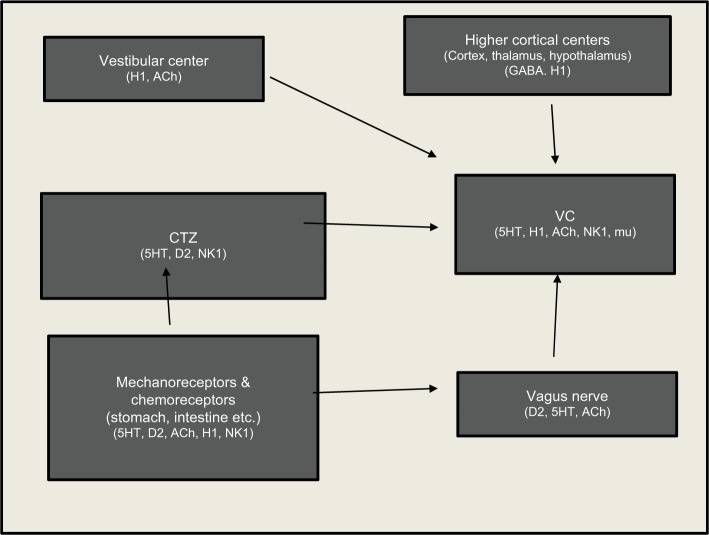
Central neurologic control of vomiting involves two anatomically and functionally separate units, the emetic center or VC and the CTZ (Figure 1). The VC consists of the nucleus tractus solitaries (NTS) and the dorsal motor nucleus of the vagus, which are both located in the medulla oblongata.13 The VC lies within the blood–brain barrier (BBB) and receives input from four areas: the CTZ, the gastrointestinal tract and other abdominal organs, the vestibular apparatus (vomiting associated with motion sickness), and the cerebral cortex (psychogenic vomiting).13 The CTZ is in the area postrema (AP) located on the dorsal surface of the medulla oblongata adjacent to the fourth ventricle. The CTZ lies outside the BBB and is responsive to circulating emetogens including drugs (such as opioids), but also uremic toxins; electrolyte, osmolar, and acid–base disorders; as well as metabolic derangements such as diabetic ketoacidosis.13 The CTZ sends signals to the VC, primarily the NTS, and the central pattern generator (CPG), which controls swallowing, gastric and lower esophageal tone and respiration and, thus, elicits the motor and autonomic responses associated with vomiting.13 NK-1 receptors and SP are present in the NTS, dorsal motor nucleus of the vagus, and the AP. NK-1 antagonists act at these sites and the CPG or the pathway between the NTS and CPG to provide broad-spectrum inhibition of vomiting caused by both peripheral and central pathways.
Figure 1.
Central neurologic control of vomiting involves two anatomically and functionally separate units; the VC or emetic center and the CTZ.
Notes: The CTZ lies outside the BBB and is responsive to circulating emetogens. These emetogenic stimuli act either peripherally by stimulating vagal or sympathetic afferents or centrally by stimulation of the CTZ. All stimuli are mediated through the VC, regardless of the cause. The VC integrates efferent input from a number of sources including the cerebral cortex (psychogenic vomiting), vestibular input arising from the semicircular canals (vomiting associated with motion sickness or vestibular disorders), vagal and sympathetic fibers, especially from the gastrointestinal system, and the CTZ. Key neurotransmitter receptors involved in the emetic response include: serotonin (5-HT), NK-1, dopamine (D2), histamine (H1), cholinergic (ACh), and mu opioid. Since stimulant pathways converge at the level of the VC, effective blocking of the vomiting reflex at this level is effective in preventing emesis, whether it is generated centrally or peripherally.
Abbreviations: ACh, acetylcholine; BBB, blood–brain barrier; CTZ, chemoreceptor trigger zone; GABA, gamma amino butyric acid; NK-1, neurokinin-1; VC, vomiting center.
Opioids can have emetic or antiemetic effects depending on the lipid solubility of the specific drug, the dose, and route of administration. The emetic effects are thought to be the result of stimulation of delta opioid receptors in the CTZ (outside the BBB), and the antiemetic effects are due to stimulation of mu receptors at the VC.14 Low doses of morphine (0.3 mg/kg IV) results in a 100% (6/6) incidence of vomiting, whereas higher doses (2.0 mg/kg) results in 0% (0/28) incidence in vomiting and also prevents vomiting induced by apomorphine.14 It is postulated that the lower dose does not cross the BBB and stimulates the CTZ, resulting in vomiting. The higher dose crosses the BBB to reach the VC and prevents vomiting. The route of administration also affects the incidence of vomiting. The incidence of vomiting for hydromorphone is highest after subcutaneous (SC) administration (75% for 0.1 mg/kg and 100% for 0.5 mg/kg) compared to 44%–66% for intramuscular (IM) doses of 0.1 mg/kg.2,20 The incidence of vomiting after IV administration of hydromorphone is 33% (3/9) for 0.1 mg/kg and 0% (0/7) for 0.5 mg/kg.3 Highly lipid soluble opioids such as fentanyl do not cause vomiting due to their effect on the VC. The incidence of vomiting at doses of 5.0 and 10 μg/kg IV was 0% (6/6 and 12/12, respectively), and the 10 μg/kg dose prevented vomiting caused by apomorphine.14
Maropitant is an NK-1 receptor antagonist which acts by inhibiting the binding of SP and blocking its pharmacologic action. It is the first drug in its class to be approved to treat and prevent vomiting specifically in dogs and cats. It been shown to significantly decrease vomiting from both centrally acting (apomorphine) and peripherally acting emetogens (syrup of ipecac).15 In the proprietary injectable formulation of maropitant (Cerenia), contains maropitant (10 mg/mL), sulphobuyether-β-cyclodextrin (SBECD, 63 mg/mL), and the preservative 3.3 mg meta-cresol (3.3 mg/mL) and water for injection.16 The cyclodextrin (SBECD) forms a molecular cavity that entraps maropitant and limits the amount of free drug, preventing direct contact with biological tissues. Cyclodextrins are used in drug formulations to improve solubility and reduce injection-related irritation without loss of therapeutic benefits.17 It is thought that the unbound maropitant is responsible for the local irritation and injection pain observed in the postmarketing surveillance of Cerenia. Narishetty et al17 found that the binding constant of the maropitant–SBECD complex exhibited an inverse relationship with temperature; as temperature increased, binding decreased, leading to more unbound maropitant and increased injection pain. There is an approximate four-fold decrease in complex binding at room temperature (22°C, 72°F) and a ten-fold decrease in binding of the complex at 37°C (98.6°F) compared to refrigerated temperatures (4°C, 39°F).16 This same study evaluated pain on injection at different temperatures. Injection pain was compared at 14°C (57°F), 22°C (72°F), and 37°C (98.6°F).17 Only 6% of dogs exhibited pain on injection in the refrigerated Cerenia group vs 26% for the room temperature and warmed Cerenia. Visual analog scales (VAS) for pain were significantly higher in dogs administered room temperature (25°C, 77°F) vs refrigerated (4°C, 39°F) Cerenia.17 Cerenia remains stable and does not degrade at room temperature; however, there is an increased likelihood for maropitant to dissociate from SCECD, producing more unbound maropitant and increased pain on injection. It is now recommended that once the vial is opened, it should be stored at refrigerated temperatures (36°F–46°F, 2°C–5°C), where there should be minimal to no free maropitant. Maropitant should be administered immediately upon removal to minimize injection pain. The increased binding of the maropitant–SBECD complex should decrease the incidence and severity of injection pain.17
The injectable maropitant (Cerenia) formulation is dosed at 1.0 mg/kg and can be administered SC in dogs aged 2–4 months for up to 5 days. In dogs 4 months of age and older, it can be also be administered slowly IV over 1–2 minutes. It should be administered at least 45–60 minutes prior to medications that may cause vomiting, including chemotherapeutic agents. In cats 4 months of age and older, 1.0 mg/kg maropitant can be administered either SC or IV slowly over 1–2 minutes once a day for up to five consecutive days. Maropitant citrate oral tablets are available in 16, 24, 60 and 160 mg. The tablets are approved for up to 5 days in dogs 2–7 months of age for the prevention of acute vomiting, and in dogs greater than 7 months of age until resolution of vomiting. In dogs that are actively vomiting, it is recommended that an initial dose of injectable maropitant be administered. Thereafter, dosing may be accomplished with either the injectable or tablet formulation. Maropitant citrate tablets are also approved for prevention of vomiting due to motion sickness in dogs 4 months of age and older. Maropitant should be dosed at a minimum of 8.0 mg/kg at least 2 hours prior to travel and for up to two consecutive days. A small amount of food should be given with the maropitant tablets to decrease vomiting and signs of nausea from the medication itself.16 The label includes a warning that the use of the injectable or tablet formulations of maropitant has not been evaluated in dogs or cats used for breeding or that are pregnant or lactating. Puppies younger than 11 weeks had evidence of bone marrow hypoplasia which may be related to NK-1 receptors presence in bone marrow and their role in hematopoisis.12 Injectable maropitant is metabolized by the cytochrome P450 enzymes CYP3A and CYPD15 in dogs and by CYP1A in cats.16 The pharmacokinetic parameters of maropitant in dogs and cats are illustrated in Tables 1 and 2, respectively.
Table 1.
Pharmacokinetics in Beagle dogs (mean ± SD)
| PK parameter | IV (1.0 mg/kg) | SC (1.0 mg/kg) | PO (2.0 mg/kg) | PO (8.0 mg/kg) |
|---|---|---|---|---|
| Cmax (ng/mL) | 1920±653 | 92±34 | 81±32 | 776±604 |
| Tmax (hr) | 0.03 | 0.75 | 1.9 | 1.7 |
| T1/2 | 6.25 | 7.75 | 4.0 | 5.5 |
Notes: The bioavailability is 91%, 24%, and 37% after SC injection of 1.0 mg/kg, 2.0 mg/kg PO, and 8.0 mg/kg PO, respectively. Data from Benchaoui et al.18
Abbreviations: Cmax, peak plasma concentration; IV, intravenous; PO, per oro; SC, subcutaneous; Tmax, time to Cmax.
Table 2.
The pharmacokinetic data from label informationa and Hickman et alb after IV, SC, and PO dose of maropitant in cats is illustrated in the table below
| Pharmacokinetic parameter | 1.0 mg/kg IV | 1.0 mg/kg SC | 1.0 mg/kg PO |
|---|---|---|---|
| Cmax (ng/mL) | 988a | 269b | 156b |
| Tmax (h) | NA | 0.43a, 0.5–2.0b | 2–3b |
| T1/2 (h) | 4.9a | 6.6a |
Notes: The reported bioavailability is 117% after SC administration and 50% after oral administration. The time to peak plasma concentration is reported to range from 26 minutes (label) up to 2 hours.19 Data from
Cerenia (tablets and injectable marketing package insert), Zoetis Inc.16 and
Hickman et al.19
Abbreviations: Cmax, peak plasma concentration; IV, intravenous; PO, per oro; SC, subcutaneous; Tmax, time to Cmax.
Treatment/prevention of perioperative vomiting and signs of nausea
Hydromorphone studies in dogs
The first study to examine maropitant as an agent to prevent perianesthetic vomiting and signs of nausea concluded that when maropitant (1.0 mg/kg SC) was administered 1 hour prior to opioid premedication, vomiting, retching, and signs of nausea were prevented.20 Eighteen dogs being admitted for elective orthopedic procedures were dosed with either saline or maropitant 1 hour prior to premedication with hydromorphone (0.1 mg/kg IM). Dogs were observed for 30 minutes for vomiting, retching, and/or signs of nausea. Vomiting was defined as expulsion of stomach contents from the mouth, whereas retching was forceful contraction of the abdominal muscles without expulsion of stomach contents. Signs interpreted as nausea included salivation, licking of lips, and increased or exaggerated swallowing motions. None of the dogs in the maropitant group vomited, retched, or exhibited signs of nausea. However, all of the dogs in the saline (placebo) group either vomited (6/9, 66%), retched (1/9, 11%), or displayed signs of nausea (2/9, 22%).20
The perceived barriers to preanesthetic treatment with maropitant to prevent opioid-induced vomiting are the waiting time for the onset of action of maropitant and also pain caused by injection. IV administration has recently been added to the Cerenia label and would speed the onset of action and avoid the pain associated with SC administration. However, IV administration has been associated with a decrease in blood pressure in healthy dogs.36 Oral administration would avoid the pain caused by SC injection and could be administered at home by the owner the evening prior to elective anesthesia. A subsequent study evaluated the effectiveness of the oral tablets in preventing vomiting and signs of nausea in dogs premedicated with hydromorphone.21 Forty dogs were dosed with either placebo or maropitant citrate tablets (2.0–4.0 mg/kg) per oral (PO) 2 hours prior to hydromorphone (0.1 mg/kg) IM. Dogs were observed for vomiting and signs of nausea using the same definitions as the previous study. In addition, the signs of nausea were subjectively graded as none, mild, moderate, or severe. Maropitant prevented vomiting, but there was no significant difference between maropitant and placebo in the incidence of signs of nausea (12/20, 60% vs 11/20, 55% respectively). In addition, 10/12 of the maropitant-treated dogs were subjectively assessed as exhibiting moderate to severe signs of nausea, whereas 14/16 of the placebo-treated dogs were assessed to have only mild clinical signs of nausea. In this study, although oral maropitant prevented vomiting, it did not prevent signs of nausea and, in fact, caused more severe signs of nausea associated with hydromorphone administration.21
Another study by Hay Kraus22 evaluated the effect of the dosing interval time on the efficacy of maropitant for prevention of vomiting and signs of nausea in dogs, as defined in previous studies. Fifty client-owned dogs were dosed with maropitant (1.0 mg/kg SC) simultaneously (time 0) or 15, 30, 45, and 60 minutes prior to administration of hydromorphone (0.1 mg/kg IM). Sixty percent (6/10) of dogs in the time 0 group vomited. Vomiting was significantly reduced by 15 minutes (2/10, 20%) and prevented in all dogs by 30 minutes. Signs of nausea were significantly reduced only at the 60 minute time interval.22 According to Benchaoui et al,18 maropitant (1.0 mg/kg SC) achieves a peak plasma concentration of 92 ng/mL at 0.75 hours. On the basis of this dosing interval study, the pharmacodynamics of the antiemetic activity of maropitant is achieved well before the time required for the drug to reach peak plasma concentration, especially when administered prior to emetic challenge. However, signs of nausea are significantly decreased only by waiting the full 60 minutes between maropitant and opioid dosing.
Claude et al23 evaluated the effects of maropitant or acepromazine on the adverse effects, including vomiting, signs of nausea, pytalism, and panting associated with hydromorphone administration in dogs. Sixty healthy female dogs scheduled for ovariohysterectomy (OHE) were dosed with saline (placebo control), maropitant (1.0 mg/kg SC), or acepromazine (0.02 mg/kg IM) 30–45 minutes prior to hydromorphone (0.2 mg/kg IM). The incidence of vomiting was none (0%), 53%, and 87% for the maropitant, acepromazine, and saline groups, respectively.23 In this study, signs of nausea were defined as excessive licking of the lips and swallowing and a hunched posture. The incidence of nausea was not significantly different between groups; (3/15 (20%), 7/15 (47%), and 9/15 (60%) for the maropitant, acepromazine, and control group, respectively). Pytalism (increased salivation) was evaluated as a separate adverse effect and was significantly increased in the maropitant group (11/15, 73%) compared to control (7/15, 47%) and acepromazine (3/15, 20%).23 The authors did not speculate on a reason for the association of increased salivation compared to other studies; however, from the dosing interval study by Hay Kraus,22 it can be noted that at least 60 minutes is required between dosing of maropitant and hydromorphone to significantly decrease signs of nausea.22 Another factor may be related to the hydromorphone dose of 0.2 mg/kg, which was twice that of the other studies. Although it is well documented that the incidence of vomiting with opioids decreases with increasing opioid dose, there is no documentation regarding the effect of dose on the incidence or severity of salivation or signs of nausea in dogs or other veterinary species.
Morphine studies in dogs
Koh et al,24 evaluated the effects of maropitant, acepromazine, and electroacupunture on the incidence of vomiting and signs of nausea associated with morphine administration in dogs. Two-hundred twenty two dogs received 1 of 6 treatments: saline, maropitant (1.0 mg/kg SC), acepromazine (0.05 mg/kg IM), or electroacupunture at either 1 acupoint (pericardium-6) or 5 acupoints (pericardium-6, stomach-36, gallbladder-34, bladder-20 and bladder 21) or a sham (non-acupoint) 20 minutes prior to morphine (0.5 mg/kg IM). In this study, the signs of nausea included ptylaism, lip-licking, swallowing, nervousness, restlessness, and signs of depression. Each of three categories of signs of nausea (lip-licking/swallowing, salivation, and attitude/mentation/posture) were scored 1 to 4 (1 = none, 4 = worst possible signs) and the mean nausea score at each time point was determined. Maropitant significantly decreased, but did not prevent vomiting and retching (14/37, 37.8% vs 28/37, 75.7% for saline group) when administered 20 minutes prior to morphine. Acepromazine significantly decreased the incidence of signs of nausea (3/37, 8.1%).24 Both acepromazine and electroacupunture appeared to help decrease the severity of signs of nausea after morphine administration.
The efficacy of maropitant in preventing vomiting and salivation caused by morphine and acepromazine premedication was investigated by Lorenzutti et al.25 Sixty female dogs admitted for elective OHE were administered either saline control or maropitant (1.0 mg/kg SC) dosed either simultaneously or 30 minutes prior to morphine (0.5 mg/kg) and acepromazine (0.05 mg/kg) IM. Maropitant had no significant effect on the incidence of vomiting or salivation when administered at the same time as morphine and acepromazine. However, when maropitant was dosed 30 minutes prior to morphine and acepromazine, the incidence of vomiting was significantly decreased but not completely prevented; 3/20 (15%) for maropitant and 10/20 (50%) for saline control. There was no significant decrease in salivation when maropitant was dosed 30 minutes prior to morphine and acepromazine.25
Lorenzutti et al26 compared the incidence of vomiting and signs of nausea in dogs administered maropitant (1.0 mg/kg) or metoclopramide (0.5 mg/kg) or saline 45 minutes prior to premedication with morphine (0.5 mg/kg) and acepromazine (0.05 mg/kg). Maropitant successfully prevented vomiting in all dogs compared to an incidence of 38% in the metoclopramide group and 71% in the saline group. However, there was no significant difference in the incidence of signs of nausea (definded as ptyalism, lip-licking, increased frequency of swallowing) between the groups. This study also documented a higher incidence of injection discomfort with maropitant (48%) compared to 9.8% for metoclopramide and 4.8% for saline.26 Ramsey et al27 also assessed the efficacy of maropitant SC to prevent vomiting and signs of nausea after morphine premedication. In addition, this study assessed the quality of recovery from anesthesia and the return to normal feeding postoperatively. Sixteen male and 16 female Beagle dogs were dosed with either maropitant or saline 45 minutes prior to premedication with morphine (0.5 mg/kg IM) followed by propofol induction and isoflurane anesthesia for routine castration or OHE. Signs of nausea included excessive salivation, increased/exaggerated swallowing, licking of lips, hunched posture, piloerection, restlessness, and vocalization. The severity of nausea signs was evaluated using a VAS where the observer placed a mark along a 100 mm line where the far left indicated no nausea and the far right indicated the worst possible nausea. None of the maropitant-treated dogs vomited compared to 15/16 of the saline-treated dogs. Maropitant significantly decreased but did not prevent signs of nausea (increased salivation, licking of the lips, restlessness, hunched posture, and vocalization) preoperatively. Maropitant-treated dogs had significantly better anesthesia recovery scores compared to saline dogs, where 25% were documented to experience a difficult or rough recovery based on behavioral observations. Postoperatively, the female dogs exhibited a significantly higher incidence of signs of nausea compared to males, which the authors attributed to the effects of intra-abdominal surgery. Females in the maropitant group exhibited less signs of nausea postoperatively compared to saline-treated females. The maropitant-treated dogs also had a faster return to feeding, with ~67% returning to feeding (consuming at least 100 gms of food) 6 hours PO compared to only 33.3% of saline-treated dogs. By 20 hours PO, 93% (all but one) of maropitant-treated dogs had returned to feeding compared to only 46% of placebo dogs. Dogs treated with maropitant also ate significantly more food, with a mean food consumption of 190 gms vs only 39 grams for saline dogs.27 This study demonstrated the many advantages of using maropitant as a preanesthetic agent, especially when used in conjunction with mu-agonist opioids, and in females undergoing OHE surgery. Maropitant prevents opioid-induced vomiting and decreases signs of nausea both preoperatively and postoperatively in females undergoing OHE surgery. Additionally, maropitant may smooth recovery from anesthesia and improve postoperative time to return to feeding and food intake, thus reversing the negative caloric energy balance associated with anesthesia and surgery and improving overall patient recovery.
Effects of maropitant on gastroesophageal reflux
Perianesthetic aspiration pneumonia has been associated with both perianesthetic vomiting and gastroesophageal reflux (GER). Anesthesia-associated GER may also lead to esophagitis and esophageal stricture. However, the mechanisms of these physiologic processes are completely different. Vomiting is defined as the forceful expulsion of gastric contents from the oral cavity and is triggered by the VC which receives input from the CTZ, as well as the gastrointestinal tract, cerebral cortex, and vestibular apparatus. GER is defined as the passive reflux of gastric fluid contents into the esophagus and a measured pH <4.0. The primary barrier to GER is the tone of the lower esophageal sphincter, which is made up of muscle layers at the gastroesophageal junction. Injectable and inhalant anesthetic drugs contribute to GER by causing relaxation of the lower esophageal sphincter. The incidence of GER has been reported as 10%–55% in dogs and as high as 50% in cats under anesthesia for dentistry procedures.28–30 Therefore, prevention of anesthesia-associated GER, in addition to vomiting, would also improve patient morbidity and mortality. Johnson evaluated the effect of IV maropitant on the incidence of vomiting and GER in dogs premedicated with hydromorphone and acepromazine.31 Twenty-six dogs admitted for elective surgical procedures were administered either saline or maropitant (1.0 mg/kg IV) 45–60 minutes prior to premedication with hydromorphone (0.1 mg/kg) and acepromazine (0.03 mg/kg) IM. The dogs were observed for vomiting/retching, and esophageal pH was monitored during inhalant anesthesia and surgery. Maropitant prevented vomiting/retching compared to saline control (0/13 vs 6/13, 46%). However, there were no significant differences between the groups for the incidence of GER (4/13 for maropitant, 6/13 for saline) or the number of reflux events.31 This is likely due to differences in the physiologic mechanisms of vomiting versus GER, but may also be due to the small study sample size.
Treatment of perioperative vomiting and signs of nausea in feline patients
Hickman et al19 performed the original safety and efficacy studies for the use of maropitant for the prevention of vomiting and motion sickness in cats. In the tolerability study, six cats were observed for their reaction to the SC injection of maropitant. Interestingly, no abnormal behavior was observed at the clinical dose of 1.0 mg/kg SC; however, the commercial formulation was not used in that study and it was not noted if the formulation was refrigerated. Maropitant administered as a single dose and for 4 days was found to be well tolerated by cats. There were no changes in behavior, appetite, or level of consciousness. Likewise, at doses up to 5.0 mg/kg administered for 15 days, no adverse effects were noted on physical exam, hematology, serum chemistry, urinalysis, or coagulation parameters. The only drug-related histopathologic findings were related to inflammation and fibrosis at the injection site. The antiemetic efficacy was confirmed using a xylazine challenge (0.44 mg/kg IM) at 2 and 24 hours post maropitant administration. Maropitant, administered at 1.0 mg/kg SC, PO, or IV decreased the mean number of vomiting events by 76%, 90%, and 100%, respectively, compared to control. At 24 hours, maropitant was effective in reducing the incidence of vomiting associated with xylazine by 66% and significantly decreased VAS nausea scores.19
As noted above, Hickman et al19 noted no abnormal behavior in cats receiving 1.0 or 2.5 mg/kg SC. However, the commercial formulation was not used in that study. Studies by the manufacturer using the commercial formulation for a supplemental drug application to add an indication for the treatment of vomiting in cats all documented moderate to marked response to SC injection characterized by vocalization, retreating, and hissing.32 In the US clinical field effectiveness study in cats, 88/133 (66%) displayed either no response or a mild response where the cat seemed aware of the injection but did not protest compared to saline control (60/62, 96.8%).32 However, a moderate response consisting of the cat objecting to the injection by retreating or vocalizing occurred in 30/133 (22.6%) of cats.32 Fifteen of 133 (11.3%) were documented to have a significant response to injection by retreating, hissing, scratching, and vocalizing.32 In the subsequent margin of safety study, assessment of behavioral responses and assessment of the severity of restraint required for the SC injection were performed.32 At the recommended dose of 1.0 mg/kg, 63% of cats had a normal behavioral response in that they seemed aware of the injection but did not protest.32 However, approximately 37% of cats were assessed to have a moderate or marked reaction to the injection, which included vocalization, scratching, or biting, and a persistent response to the injection site.32 Other behaviors associated with injection included licking, scratching, or biting at the injection site, growling, urination, salivation, and vomiting.32 Approximately half of the cats required moderate to substantial increase in manual restraint of the cat and/or use of a cat bag or other safety equipment.32
Martin-Flores et al33 were the first to evaluate the use of maropitant for prevention and treatment of perianesthetic vomiting and signs of nausea in cats. In that study, 66 cats were treated with either maropitant (1.0 mg/kg SC) or saline 20 hours prior to premedication with dexmedetomidine (20 μg/kg) and morphine (0.1 mg/kg) IM. Blinded observers evaluated the cats for vomiting/retching, signs of nausea (defined as sialorrhea, excessive lip-licking), and the response to injection. Aversive behavior in response to injection was evaluated using a VAS where the observer placed a mark over a 10 cm straight line on which the left aspect of the line indicated no response and the right represented the most adverse reaction possible. Maropitant significantly decreased vomiting (1/32, 3.0%) compared to saline (20/34, 59%). Maropitant also significantly decreased, but did not prevent, retching (6/32, 19%) compared to saline (19/34, 56%). Signs of nausea were decreased (11/32, 34% vs 19/34, 56%) but did not reach statistical significance. The VAS scores for cats receiving maropitant SC were significantly higher than the cats receiving saline. In fact, over 27% of cats in the maropitant group were assigned a VAS score of 7 or greater, compared to only 4% of the saline cats. The aversive behaviors consisted primarily of vocalization and attempts to escape restraint. One cat in the maropitant group received a VAS score of ten, indicating the worst reaction imaginable to an SC injection.33 To the author’s knowledge, there are no studies on the incidence of perioperative aspiration pneumonia in cats. However, one may assume that, like canine patients, the incidence may be low but mortality high and may be associated with many of the same risk factors, including perianesthetic vomiting. However, the dilemma does become one of whether pretreating all cats with SC maropitant and causing significant discomfort is justified for decreasing perianesthetic vomiting and possible aspiration.
Due to concern over patient discomfort with SC maropitant injection, the same investigators evaluated the efficacy of the oral maropitant formulation in preventing perianesthetic vomiting and signs of nausea.34 Signs of nausea included licking of the lips or sialorrhea, which was identified as clear or frothy fluid around the lips with or without dripping. Ninety-eight cats were administered 8.0 mg (mean dose 2.5 mg/kg) PO 18 hours prior to premedication with dexmedetomidine (20 μg/kg) and morphine (0.1 mg/kg) IM. This was meant to simulate dosing the night before a planned anesthetic procedure. Maropitant significantly decreased, but did not completely prevent, vomiting (2/46, 4%) compared to the control group (20/50, 40%). Maropitant also significantly decreased the incidence of retching in cats (4/46, 8% vs 20/50, 40%). As in dogs, oral maropitant did not significantly decrease the incidence of sialorrhea (10/46, 21% for maropitant compared to 11/50, 22% for control). However, the maropitant group did have a significant decrease in the incidence of lip-licking (14/46, 30% vs 26/50, 52% for control), which may be interpreted as one of the signs of nausea in dogs and cats.34
Martin-Flores et al35 also evaluated the efficacy of oral maropitant dosed 2.0–2.5 hours prior to the same premedication protocol to simulate administration the morning of the anesthetic procedure. Eighty-three cats were administered 8.0 mg (mean dose 2.9 mg/kg) PO prior to dexmedetomidine (20 μg/kg) and morphine (0.1 mg/kg) IM. As in the previous study, maropitant significantly decreased but did not prevent the incidence of vomiting and retching. Vomiting occurred in 5/39 (13%) of maropitant-treated cats compared to 14/44 (32%) of control cats. Likewise, retching occurred in 5/39 (13%) of maropitant cats and 16/44 (36%) of control cats. In this study, maropitant did not decrease sialorrhea or lip-licking. In fact, maropitant-treated cats experienced a higher incidence of sialorrhea (8/38, 21%) compared to control cats (4/43, 9%).35 Similar to dogs, oral maropitant is more effective as an antiemetic agent than antinausea agent.
It is clear from both the initial safety/tolerability studies and subsequent clinical studies that maropitant dosed SC or PO is effective in decreasing but not completely preventing vomiting/retching in cats and provides minimal protection against signs of nausea defined as increased salivation and/or lip-licking. It may be more efficacious if dosed IV prior to an emetic challenge. Cats may be more similar to human patients in that monotherapy is less successful in the prevention of perianesthetic nausea and vomiting, and efforts should include choice of drug protocols associated with lower incidence of nausea and vomiting.
Adjunct analgesia
NK-1 receptors and SP are found in multiple areas of the pain pathways, including sensory afferents, dorsal root ganglia, dorsal horn, and ascending projections of the spinal cord and higher brain centers involved in pain perception. NK-1 receptors have been identified in the dorsal horn of the spinal cord in cats and in viscera such as the esophagus, colon, and urinary bladder in rats.36 Greater than 80% of visceral afferents contain the SP neuropeptide versus only 21% of somatic afferents, suggesting a greater role for NK1 receptor antagonists in visceral antinociception than for somatic.37
Although clinical trials in humans, especially for treatment of somatic type pain, have not consistently shown efficacy, numerous animal models have demonstrated efficacy for visceral analgesia. NK-1 receptor antagonists have been shown to be effective for visceral pain in a number of laboratory animal models such as noxious bladder and colonic stimulation in mice and guinea pigs and colorectal stimulation in rabbits.36 Boscan et al36 used a validated canine laparoscopic ovarian pedicle stimulation model to evaluate the effect of maropitant on anesthetic requirements. Dogs were anesthetized with sevoflurane and administered maropitant (1.0 mg/kg IV followed by 30 μg/kg/hr IV). Maropitant decreased the minimal alveolar inhalant anesthetic requirements (MAC) by 24% during visceral stimulation of the ovary and ovarian ligament. Similar results were found in cats, with maropitant (1.0 mg/kg IV) decreasing the MAC of sevoflurane by 15% using a similar ovarian stimulation model.37 However, in both studies and species, a higher dose of 5.0 mg/kg did not lead to a significant further decrease in the MAC of sevoflurane. A subsequent study used a tail clamp stimulation model in dogs to simulate stimulation of the somatic tissues of bone, skin, and soft tissues. Maropitant (5.0 mg/kg IV) decreased the MAC of sevoflurane by 16%.38 However, the MAC did not significantly change significantly when the maropitant (1.0 mg/kg) was administered via an epidural catheter.38
Marquez et al39 performed a blinded clinical trial comparing maropitant (1.0 mg/kg) and morphine (0.5 mg/kg) SC in healthy female dogs admitted for elective OHE. Dogs were administered morphine or maropitant 30 minutes prior to induction with propofol followed by inhalant anesthesia. Dogs were monitored for heart rate, systolic blood pressure, end-tidal isoflurane concentration, pain assessment via VAS and Colorado Acute Pain Scale (CSU), recovery quality, and return to feeding. Dogs in the maropitant group had lower heart rates, systolic blood pressure, and inhalant anesthetic requirement compared to dogs receiving morphine. At extubation, maropitant-treated dogs had significantly lower VAS and CSU pain scores. However, there was no significant difference in pain scores at any subsequent time point and no significant difference in the requirement for rescue analgesia between groups. Maropitant-treated dogs were significantly more likely to eat within 3 hours of extubation (65%) compared to only 15% in the morphine group.39
Fukui et al40 evaluated the effect of maropitant, carprofen, or the combination of the two on the minimum alveolar concentration for blunting the adrenergic response (MAC-BAR) of sevoflurane in dogs. The MAC-BAR is the minimum anesthetic concentration which prevents an autonomic response to noxious stimulus and provides information related to intraoperative neuroendocrine stress, whereas traditional MAC reflects suppression of motor neurons in the ventral horn of the spinal cord. Six Beagle dogs were dosed with either maropitant (1.0 mg/kg) or carprofen (4.0 mg/kg) alone, or both maropitant and carprofen or saline 1 hour prior to MAC-BAR determination. MAC-BAR was measured during anesthesia with sevoflurane by determining the response to a noxious electrical stimulus (50 Hz, 10 msec) applied to the gingiva using an electrical stimulator. In this study, MAC-BAR was significantly reduced with maropitant and carprofen alone and the combination compared with saline. There was no significant difference in the mean percentage of MAC-BAR of sevoflurane reduction between maropitant (15%), carprofen (10.2%), or the combination (16.2%), indicating a lack of additive effect of the two drugs.40 This study helps to further elucidate the roles of these two agents in providing analgesia. Maropitant provides analgesia by blocking the pharmacologic effect of SP at the spinal cord and brain through its antagonism at the NK-1 receptors. Nonsteroidal anti-inflammatory drugs (NSAIDs), such as carprofen produce peripheral analgesia and anti-inflammatory effects by inhibiting prostaglandin production via inhibition of cyclooxygenase. However, there is increasing evidence that NSAIDs also have antinociceptive effects in the central nervous system by decreasing prostaglandin facilitated release of SP from central C-fiber nerve terminals. It was postulated that, in this study, inhibition of SP release from the C-fibers by carprofen did not further reduce MAC-BAR since maropitant had already blocked binding of Substance P to NK-1 receptors in the spinal cord. Maropitant may provide similar central analgesia to NSAIDs both in MAC-sparing capability and central nociceptive pathways but provide a more favorable side effect profile than NSAIDs.
Clinical recommendations
From the body of research and clinical studies above, there is a preponderance of evidence of the advantages of using maropitant as a preanesthetic agent in both dogs and cats, especially when used in conjunction with mu-agonists and in females undergoing OHE surgery. Maropitant prevents opioid-induced vomiting and decreases signs of nausea pre-op and post-op in females undergoing OHE surgery. For canine patients, 1.0 mg/kg SC administered 1 hour prior to hydromorphone has been shown to prevent vomiting and signs of nausea. To date, the studies evaluating the effectiveness of maropitant prior to morphine premedication have only allowed 20–45 minutes after maropitant administration, resulting in a significant decrease, but not complete elimination of, vomiting and signs of nausea. Since morphine is less lipid soluble than hydromorphone and may be a stronger emetogen than hydromorphone, the recommendation would be to allow the full 1 hour prior to morphine administration to allow full onset of action of maropitant SC. To minimize injection pain, opened vials of maropitant should be stored at refrigerated temperatures (36°F–46°F, 2°C–5°C) and administered immediately. Oral dosing (2.0–2.5 mg/kg) of maropitant in dogs 2 hours prior to hydromorphone administration is effective in preventing vomiting; however, it does not prevent signs of nausea and appears to actually increase the severity of visible signs of nausea, including salivation, licking of lips, and increased swallowing.
Subcutaneous administration of maropitant in cats is effective in decreasing, but not eliminating, vomiting and signs of nausea prior to morphine/dexmedetomidine premedication. However, feline patients exhibit significant aversive behaviors and pain upon SC injection, and therefore oral administration at least 2–3 hours prior to opioid premedication may be a more humane option. Oral maropitant significantly decreases but does not eliminate vomiting and signs of nausea associated with morphine/dexmedetomidine premedication in cats. Currently, there are no studies evaluating the effect of refrigeration of maropitant on injection pain in cats.
IV administration has recently been added to the label specifictions. Peak plasma concentrations occur within minutes of IV injection, and therefore should effectively prevent vomiting and signs of nausea related to opioid administration. However, to date, no dosing interval studies have been completed assessing effectiveness prior to emetogen/opioid challenge. The label recommends IV administration slowly over 1–2 minutes. This will help to avoid or ameliorate hypotension associated with IV administration. However, the author would also recommend monitoring of blood pressure during and after IV administration, especially in nonhealthy or critically ill patients.
Additional documented advantages to adding maropitant to perianesthetic regimes are smoother recovery from anesthesia, adjunct analgesia, especially in female patients for OHE, and inhalant MAC sparing. Dogs treated with maropitant also return to feeding earlier and eat more food postoperatively, helping to speed recovery and healing and reversing the negative energy balance often associated with surgery and anesthesia. Smooth recovery and faster return to eating may allow improved overall patient recovery, earlier discharge, decreased expense, and increased owner satisfaction. Lastly, canine owners are concerned about their pets experiencing nausea and vomiting associated with anesthesia and pain medications and are willing to pay for effective prevention.
Footnotes
Disclosure
The author reports no conflicts of interest in this work.
References
- 1.Gan TJ, Diemunsch P, Habib AS, et al. Consensus guidelines for the management of postoperative nausea and vomiting. Anesth Analg. 2014;118:85–113. doi: 10.1213/ANE.0000000000000002. [DOI] [PubMed] [Google Scholar]
- 2.Valverde A, Cantwell S, Hernandez J, Brotherson C. Effects of acepromazine on the incidence of vomiting associated with opioid administration in dogs. Vet Anaesth Analg. 2004;31:15–22. doi: 10.1111/j.1467-2995.2004.00128.x. [DOI] [PubMed] [Google Scholar]
- 3.KuKanich B, Hogan BK, Krugner-Higby LA, Smith LJ. Pharmacokinetics of hydromorphone hydrochloride in healthy dogs. Vet Anaesth Analg. 2008;35(3):256–264. doi: 10.1111/j.1467-2995.2007.00379.x. [DOI] [PubMed] [Google Scholar]
- 4.Kogan DA, Johnson LR, Sturges BK, Jandrey KE, Pollard RE. Etiology and clinical outcome in dogs with aspiration pneumonia: 88 cases (2004–2006) J Am Vet Med Assoc. 2008;233(11):1748–1755. doi: 10.2460/javma.233.11.1748. [DOI] [PubMed] [Google Scholar]
- 5.Alwood AJ, Brainard BM, LaFond E, Drobatz KJ, King LG. Postoperative pulmonary complications in dogs undergoing laparotomy: frequency, characterization and disease-related risk factors. J Vet Emerg Crit Care. 2006;16:176–183. [Google Scholar]
- 6.Tart KM, Babski DM, Lee JA. Potential risks, prognostic indicators and diagnostic and treatment modalities affecting survival in dogs with presumptive aspiration pneumonia: 125 cases (2005–2008) J Vet Emerg Crit Care. 2010;20:319–329. doi: 10.1111/j.1476-4431.2010.00542.x. [DOI] [PubMed] [Google Scholar]
- 7.Ovbey DH, Wilson DV, Bednarski RM, et al. Prevalence and risk factors for canine post-anesthetic aspiration pneumonia (1999–2009): a multicenter study. Vet Anaesth Analg. 2014;41(2):127–136. doi: 10.1111/vaa.12110. [DOI] [PubMed] [Google Scholar]
- 8.Macario A, Weinger M, Truong P, Lee M. Which clinical anesthesia outcomes are both common and important to avoid? The perspective of a panel of expert anesthesiologists. Anesth Analg. 1999;88:1085–1091. doi: 10.1097/00000539-199905000-00023. [DOI] [PubMed] [Google Scholar]
- 9.Lehmann M, Monte K, Barach P, Kindler CH. Postoperative patient complaints: a prospective interview study of 12,276 patients. J Clin Anesth. 2010;22:13–21. doi: 10.1016/j.jclinane.2009.02.015. [DOI] [PubMed] [Google Scholar]
- 10.Morton DB, Berghardt GM, Smith JA. Animals, science and ethics – section III. Critical anthropomorphism, animal suffering and the ecological context. Hastings Cent Rep. 1990;20(3):S13–S19. [PubMed] [Google Scholar]
- 11.Hay Kraus B, Cazlan C. Assessment of dog owner concern regarding perioperative nausea and vomiting and willingness to pay for antiemetic treatment. Vet Anaesth Analg. 2015;42:A58. doi: 10.3389/fvets.2019.00264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garcia-Recio S, Gascon P. Biological and pharmalogical aspects of the NK1-Receptor. BioMed Res Internat. 2015;495704 doi: 10.1155/2015/495704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elwood C, Devauchell P, Elliott V, et al. Emesis in dogs: a review. J Small Anim Pract. 2010;51(1):4–22. doi: 10.1111/j.1748-5827.2009.00820.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blancquaert JP, Lefebvre RA, Willems JL. Emetic and antiemetic effects of opioids in the dog. Eur J Pharmacol. 1986;128:143–150. doi: 10.1016/0014-2999(86)90760-0. [DOI] [PubMed] [Google Scholar]
- 15.Sedlacek HS, Ramsey DS, Boucher JF, Eagleson JS, Conder GA, Clemence RG. Comparative efficacy of maropitant and selected drugs in preventing emesis induced by centrally or peripherally acting emetogens in dogs. J Vet Pharmacol Ther. 2008;31:533–537. doi: 10.1111/j.1365-2885.2008.00991.x. [DOI] [PubMed] [Google Scholar]
- 16.Cerenia (tablets and injectable marketing package insert) Kalmazoo, MI: Zoetis Inc; 2015. [Google Scholar]
- 17.Narishetty ST, Galvan B, Coscarelli E, et al. Effect of refrigeration of the antiemetic Cerenia (maropitant) on pain on injection. Vet Ther. 2009;10(3):93–102. [PubMed] [Google Scholar]
- 18.Benchaoui HA, Cox SR, Schneider RP, Boucher JF, Clemence RG. The pharmacokinetics of maropitant, a novel neurokinin-1 antagonist, in dogs. J Vet Pharmacol Ther. 2007;30:336–344. doi: 10.1111/j.1365-2885.2007.00877.x. [DOI] [PubMed] [Google Scholar]
- 19.Hickman MA, Cox SR, Mahabir S, et al. Saftey, pharmacokinetics and use of the novel NK-1 receptor antagonist maropitant (Cerenia) for the prevention of emesis and motion sickness in cats. J Vet Pharmacol Therap. 2008;31:220–229. doi: 10.1111/j.1365-2885.2008.00952.x. [DOI] [PubMed] [Google Scholar]
- 20.Hay Kraus BL. Efficacy of maropitant in preventing vomiting in dogs premedicated with hydromorphone. Vet Anaesth Analg. 2013;40(1):28–34. doi: 10.1111/j.1467-2995.2012.00788.x. [DOI] [PubMed] [Google Scholar]
- 21.Hay Kraus BL. Efficacy of orally administered maropitant citrate in preventing vomiting associated with hydromorphone administration in dogs. J Am Vet Med Assoc. 2014;244(10):1164–1169. doi: 10.2460/javma.244.10.1164. [DOI] [PubMed] [Google Scholar]
- 22.Hay Kraus BL. Effect of dosing interval on efficacy of maropitant for prevention of hydromorphone-induced vomiting and signs of nausea in dogs. J Am Vet Med Assoc. 2014;245(9):1015–1020. doi: 10.2460/javma.245.9.1015. [DOI] [PubMed] [Google Scholar]
- 23.Claude AK, Dedeaux A, Chiavaccini L, Hinz S. Effects of maropitant citrate or acepromazine on the incidence of adverse events associated with hydromorphone premedication in dogs. J Vet Intern Med. 2014;28(5):1414–1417. doi: 10.1111/jvim.12414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koh RB, Isaza N, Xie H, Cooke K, Robertson SA. Effects of maropitant, acepromazine, and electroacupuncture on vomiting associated with administration of morphine in dogs. J Am Vet Med Assoc. 2014;244(7):820–829. doi: 10.2460/javma.244.7.820. [DOI] [PubMed] [Google Scholar]
- 25.Lorenzutti AM, Martín-Flores M, Litterio NJ, Himelfarb MA, Zarazaga MP. Evaluation of the antiemetic efficacy of maropitant in dogs medicated with morphine and acepromazine. Vet Anaesth Analg. 2016;43(2):195–198. doi: 10.1111/vaa.12286. [DOI] [PubMed] [Google Scholar]
- 26.Lorenzutti AM, Martín-Flores M, Litterio NJ, Himelfarb MA, Invaldi SH, Zarazaga MP. A comparison between maropitant and metoclopramide for the prevention of morphine-induced nausea and vomiting in dogs. Can Vet J. 2017;58(1):35–38. [PMC free article] [PubMed] [Google Scholar]
- 27.Ramsey D, Fleck T, Berg T, et al. Cerenia prevents perioperative nausea and vomiting and improves recovery in dogs undergoing routine surgery. Intern J Appl Res Vet Med. 2014;12(3):228–237. [Google Scholar]
- 28.Torrente C, Vigueras I, Manzanilla EG, et al. Prevalence of and risk factors for intraoperative gastroesophageal reflux and postanesthetic vomiting and diarrhea in dogs undergoing general anesthesia. J Vet Emerg Crit Care. 2017 May 23; doi: 10.1111/vec.12613. Epub. [DOI] [PubMed] [Google Scholar]
- 29.Wilson DV, Evans AT, Mauer WA. Pre-anesthetic meperidine: associated vomiting and gastroesophageal reflux during the subsequent anesthetic in dogs. Vet Anaesth Analg. 2007;34:15–22. doi: 10.1111/j.1467-2995.2006.00295.x. [DOI] [PubMed] [Google Scholar]
- 30.Garcia RS, Belafsky PC, Della Maggiore A, et al. Prevalence of gastroesophageal reflux in cats during anesthesia and effect of omeprazole on gastric pH. J Vet Intern Med. 2017;31:734–742. doi: 10.1111/jvim.14704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johnson R. Maropitant prevented vomiting but not gastroesophageal reflux in anesthetized dogs premedicated with acepromazine-hydromorphone. Vet Anaesth Analg. 2014;41(4):406–410. doi: 10.1111/vaa.12120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Freedom of Information Summary. Supplemental new animal drug application; to add ‘treatment of vomiting in cats. 2012. pp. 1–18. (NADA 141–263). [Google Scholar]
- 33.Martin-Flores M, Sakai DM, Learn MM, et al. Effects of maropitant in cats receiving dexmedetomidine and morphine. J Am Vet Med Assoc. 2016;248(11):1257–1261. doi: 10.2460/javma.248.11.1257. [DOI] [PubMed] [Google Scholar]
- 34.Martin-Flores M, Sakai DM, Mastrocco A, et al. Evaluation of oral maropitant as an antiemetic in cats receiving morphine and dexmedetomidine. J Feline Med Surg. 2016;18(11):921–924. doi: 10.1177/1098612X15613389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martin-Flores M, Mastrocco A, Lorenzutti AM, et al. Maropitant administered orally 2–2.5 h prior to morphine and dexmedetomidine reduces the incidence of emesis in cats. J Feline Med Surg. 2017;19(8):876–879. doi: 10.1177/1098612X16663595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boscan P, Monnet E, Mama K, Twedt DC, Congdon J, Steffey EP. Effect of maropitant, a neurokinin 1 receptor antagonist, on anesthetic requirements during noxious visceral stimulation of the ovary in dogs. Am J Vet Res. 2011;72(12):1576–1579. doi: 10.2460/ajvr.72.12.1576. [DOI] [PubMed] [Google Scholar]
- 37.Niyom S, Boscan P, Twedt DC, Monnet E, Eickhoff JC. Effect of maropitant, a neurokinin-1 receptor antagonist, on the minimum alveolar concentration of sevoflurane during stimulation of the ovarian ligament in cats. Vet Anaesth Analg. 2013;40(4):425–431. doi: 10.1111/vaa.12017. [DOI] [PubMed] [Google Scholar]
- 38.Alvillar BM, Boscan P, Mama KR, Ferreira TH, Congdon J, Twedt DC. Effect of epidural and intravenous use of the neurokinin-1 (NK-1) receptor antagonist maropitant on the sevoflurane minimum alveolar concentration (MAC) in dogs. Vet Anaesth Analg. 2012;39(2):201–205. doi: 10.1111/j.1467-2995.2011.00670.x. [DOI] [PubMed] [Google Scholar]
- 39.Marquez M, Boscan P, Weir H, Vogel P, Twedt DC. Comparison of NK-1 receptor antagonist (Maropitant) to morphine as a pre-anaesthetic agent for canine ovariohysterectomy. PLoS One. 2015;10(10):e0140734. doi: 10.1371/journal.pone.0140734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fukui S, Ooyama N, Tamura J, et al. Interaction between maropitant and carprofen on sparing of the minimum alveolar concentration for blunting adrenergic response (MAC-BAR) of sevoflurane in dogs. J Vet Med Sci. 2017;79(3):502–508. doi: 10.1292/jvms.15-0666. [DOI] [PMC free article] [PubMed] [Google Scholar]