Abstract
Feline injection site sarcomas (FISS; also known as vaccine-associated sarcomas) have been recognized for >20 years. Although uncommon, these tumors are iatrogenic, and vaccination against rabies and feline leukemia virus is perhaps the most common inciting cause. The exact etiopathogenesis is unknown, but it is widely accepted that inflammation induced by vaccines or other injections likely plays a critical role in tumor development. Injection site sarcomas are extremely locally invasive. Multimodal therapy, incorporating combinations of surgery, radiation therapy, and sometimes chemotherapy or immunotherapy, is recommended. However, tumor recurrences are common even with aggressive treatment, and many cats with FISS ultimately succumb to this devastating disease. While vaccination protocols play an important role in the management and control of infectious disease, veterinarians must be diligent in following established vaccination guidelines to minimize individual patient risk of FISS development. Early tumor detection and client education are also vital in the successful treatment of FISS.
Keywords: injection site sarcoma, cat, cancer, oncology
Background, epidemiology, and pathogenesis
Feline injection site sarcomas (FISS) have been recognized since the early 1990s. Contemporaneous with the implementation of stricter vaccination recommendations and the development of adjuvanted killed rabies and feline leukemia virus (FeLV) vaccines, pathologists at the University of Pennsylvania began recognizing an increase in the incidence of vaccine reactions.1 More importantly, they also noted an increase in the development of sarcomas at vaccination sites.2,3 Over the past 2 decades, this problem has been recognized worldwide.4–6
Subsequent investigation into the etiopathogenesis of FISS has led to the hypothesis that these tumors are induced secondary to a chronic and robust inflammatory response to the vaccine or injection, with ultimate malignant transformation of surrounding fibroblasts and myofibroblasts.7,8 This theory is supported by the characteristic histological appearance of FISS, which includes the presence of increased numbers of inflammatory cells (predominantly lymphocytes), multinucleated giant cells, central areas of necrosis, and in some cases, a grayish-blue material within macrophages, consistent with aluminum-based vaccine adjuvant.1,3,7,9,10 While the exact cause and effect have not been entirely elucidated, it has been widely theorized that the individual cat’s inflammatory response mounted and the characteristics of the vaccine (or injection) play roles in the development of these tumors.2,7,8,11–13
A significant correlation between vaccination with rabies and/or FeLV vaccines and development of FISS has been documented.3,11,12 Additionally, it has been shown that the risk of FISS development increases with the number of vaccines administered per site. Specifically, when vaccines are administered in the interscapular area, the risk of FISS development is ~50% greater than in cats not receiving vaccines; this risk increases to >127% and to >175% with administration of two and three to four vaccines, respectively.12 In 2003, results from a prospective multicenter case–control study examining risk factors associated with FISS development were published. No specific type or manufacturer of vaccine was implicated, and factors associated with vaccination practices including needle gauge, syringe type, use of multidose vaccine vials, and mixing of vaccines in one syringe did not appear to factor into risk. In fact, the temperature of the vaccine was the only potential risk factor identified, suggesting that vaccines should be warmed to room temperature prior to administration.14 Although the aluminum-based adjuvant in vaccines has often been blamed for a contributing role in FISS development,3,15 this particular study failed to prove that hypothesis.14 With that being said, more studies are needed to exactly determine the specific risk factors for FISS development.
Since the recognition of FISS, epidemiological studies have estimated an incidence of one in 1,000 to 0.63 in 10,000 cats vaccinated.12,16–18 Vaccines are most commonly linked to FISS, but other injections and implants including long-acting steroids and antibiotics,14,19 nonabsorbable suture material,20,21 and microchips22 have been implicated, leading to the name change from vaccine-associated sarcoma to injection site sarcoma. Latency periods between vaccine administration and tumor development range from 2 months to 10 years.23,24
FISS are mesenchymal in origin.7 They are extremely locally invasive, much more so than their less common, non-vaccine-associated sarcoma counterparts.11 The reported metastatic rate is 10%–25% with common metastatic sites including lungs and regional lymph nodes.17,25–28 Fibrosarcoma is most commonly diagnosed, but other reported histological types include malignant fibrous histiocytoma, rhabdomyosarcoma, myxosarcoma, liposarcoma, nerve sheath tumor, poorly differentiated sarcomas, and extraskeletal osteosarcoma and chondrosarcoma.7,25,29,30 In the author’s opinion, any sarcoma occurring in the vicinity of any potential site of vaccination or injection should be considered an FISS and treated aggressively. Depending upon the location of the injection, the angle of the needle during administration, and the subsequent tracking along fascial planes of the vaccine or injected material, sarcomas may occur at sites including (but not limited to) the interscapular area, over the scapulae or thorax, over the hips or pelvic limbs, and along the caudal dorsum and ventral abdomen.31
Diagnosis and staging
When a cat is presented for a subcutaneous mass, it is important to consider the history. Points to note include the cat’s vaccination history, the location of the mass, when it was first noticed, any change in size, and the current size of the mass (measured with calipers; Figure 1). If FISS is even remotely possible, indications for biopsy are based on the 3-2-1 rule.13,32,33 Any mass that has persisted for >3 months, is >2 cm, and/or is growing over the course of 1 month since an injection was given at that site, an incisional biopsy is strongly recommended. Fine needle aspiration with cytology is the least invasive and least expensive form of incisional biopsy. Although it does not always yield a definitive diagnosis of FISS, cytology can be helpful in ruling in or out other differential diagnoses, such as abscesses. Alternative forms of incisional biopsy including needle core, punch, and wedge biopsies may be considered. Because FISS are heterogeneous masses, multiple samples from different areas should be collected to ensure definitive diagnosis, especially when using less invasive biopsy techniques (eg, needle core or punch biopsy). When performing an incisional biopsy, it is imperative to control the risk of tumor seeding by minimizing bleeding and containing biopsy tracts within future radiation and/or surgical fields (Figure 2). Placement of surgical drains should also be avoided as they can become contaminated with and track neoplastic cells. If FISS is suspected, excisional biopsy for diagnostic purposes is strongly discouraged, as tumors recur quickly and frequently when marginally excised, making future attempts at treatment challenging.13,23,24,32,33
Figure 1.
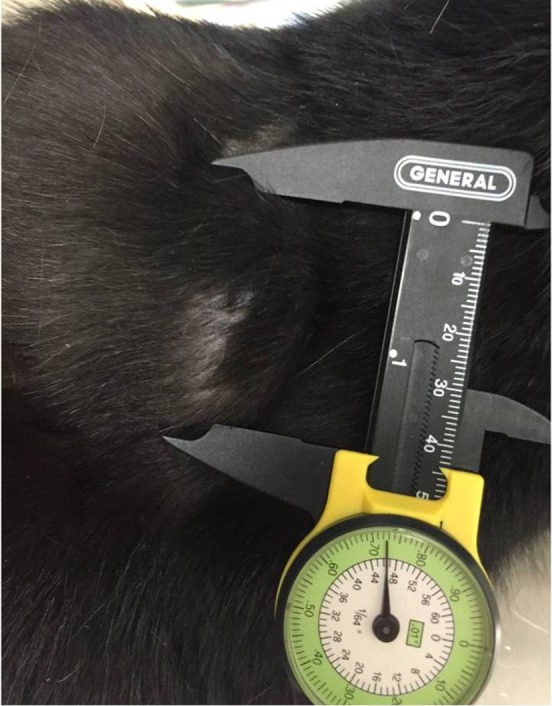
Tumor measurement with calipers.
Notes: Because of the size of this mass (4.4 cm), an incisional biopsy was recommended. Photo courtesy of Dr Nicole Northrup.
Figure 2.
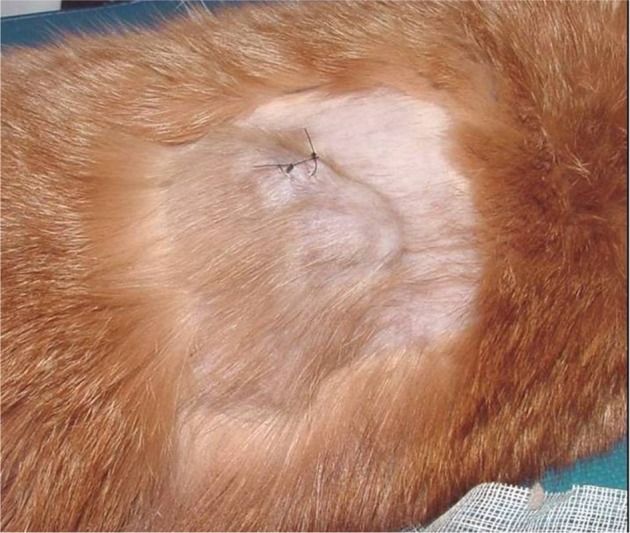
Incisional biopsy of FISS.
Notes: The location of the biopsy was strategically placed such that it was contained within future radiation and/or surgical fields. Photo courtesy of Dr Nicole Northrup.
Abbreviation: FISS, Feline injection site sarcomas.
Once FISS has been confirmed, thoracic radiographs are recommended to look for evidence of pulmonary metastatic disease. Additionally, advanced imaging (most commonly computed tomography scan as in Figure 3, but occasionally magnetic resonance imaging) is encouraged for radiation and/or surgical planning.23,34 Complete blood count, serum biochemical profile, urinalysis, T4, and FeLV/feline immunodeficiency virus (FIV) testing are often performed as part of the patient’s minimum database. However, often these tests are normal or indicative of comorbidities unrelated to the FISS.23 A link between FeLV or FIV infection and FISS development has not been made.23,35
Figure 3.
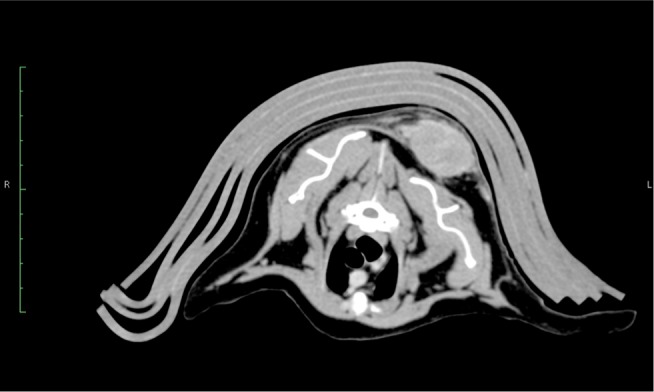
CT image of a 14 year old FS DSH with an interscapular FISS, just dorsomedial to the left scapula.
Note: Photo courtesy of Dr Christopher Brouwer.
Abbreviations: CT, computed tomography; FISS, Feline injection site sarcomas; DSH, domestic short hair.
Treatment
Although wide or radical surgical excision (defined as 3–5 cm of grossly normal tissue in all directions around the tumor and one to two fascial planes deep to it) is the mainstay of treatment for eliminating macroscopic FISS, cures remain uncommon.24,25,36,37 Most tumors will recur locally, especially when treated with surgery alone, with reported median times to recurrence ranging from 2 months to >16 months.25,37 Recurrences often occur at multiple sites along the surgical scar as seen in Figures 4 and 5. Cats with tumors located distally enough on their limbs or tails, where amputation or hemipelvectomy results in wide microscopic surgical margins, are potential candidates for successful treatment with surgery alone.25,38 However, in reality, these tumors must be located at or distal to the hock or distal third of the antebrachium or tail. Phelps et al36 reported a 14% local recurrence rate in cats treated with radical excision, defined as 5 cm margins and two fascial planes deep. However, these results must be interpreted with caution as over one-third of the cats (35%) were censored from analysis due to lost to follow-up prior to documentation of local recurrence.36 When surgical excision is performed, the specimen margins should be inked or identified with suture. The entire specimen should be adequately fixed in formalin and submitted en bloc to a commercial veterinary pathology laboratory for histopathological examination.
Figure 4.
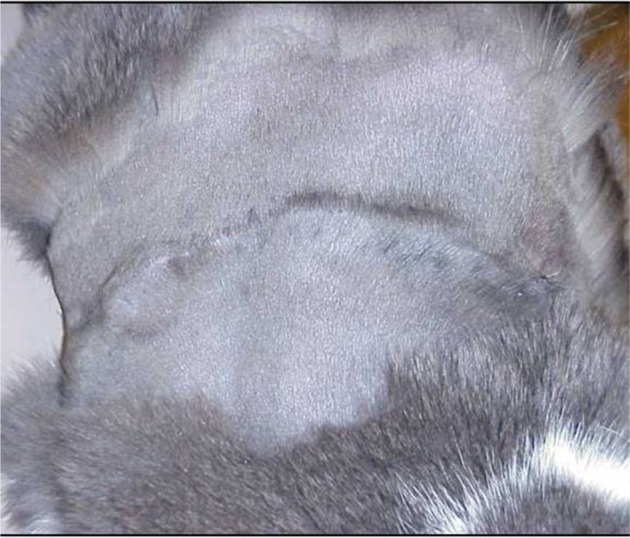
Multifocal tumor regrowth following surgical excision of FISS.
Notes: Note that there are masses along the entire length of the scar. Photo courtesy of Dr Nicole Northrup.
Abbreviation: FISS, Feline injection site sarcomas.
Figure 5.
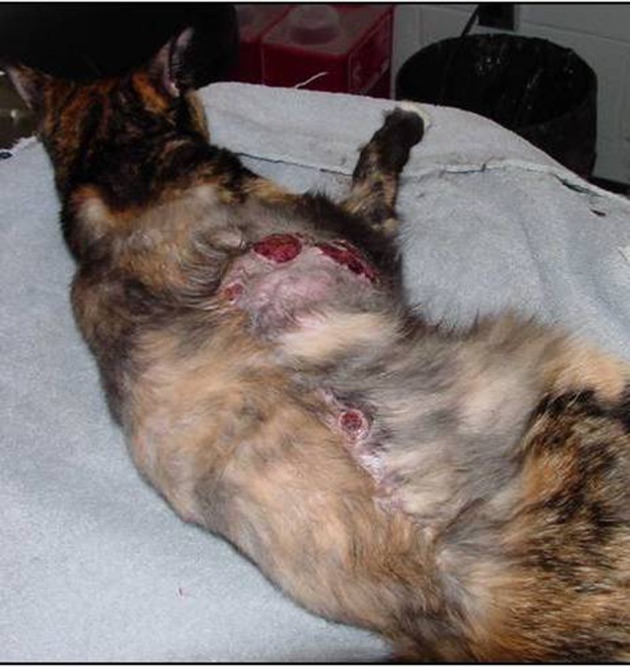
Multifocal tumor regrowth with ulceration following surgery.
Notes: Note that there are masses along the entire length of the scar. Photo courtesy of Dr Nicole Northrup.
In an attempt to prevent local tumor recurrence or at least prolong the disease-free interval (DFI), multimodal treatment including surgery and definitive radiation therapy is often recommended. Pre- and postoperative radiation protocols have been described with pros and cons to both.27,39–43 When delivered preoperatively, the entire tumor plus a wide margin (3–5 cm) of normal tissues around the tumor is treated with radiation, and surgical excision is performed 2–4 weeks following completion of the radiation protocol. Figure 6A and B shows 3D images of a cat’s preoperative radiation plan, demonstrating the large field of tissue that must be irradiated. With that being said, treatment fields are typically smaller with the preoperative radiation approach, exposing less of the normal surrounding tissues to radiation. Also, with preoperative radiation therapy, the normal blood supply to the tumor is preserved, ensuring that cells at the periphery are well-oxygenated and, therefore, more radiosensitive. A con to this approach is the increased risk of postoperative wound dehiscence as the irradiated skin is not normal and more prone to delayed or impaired healing. When delivered postoperatively, the entire surgical scar plus a wide margin (3–5 cm) of normal tissues around the scar is treated once the surgical site has healed. The advantage of postoperative radiation therapy is that surgery can occur as soon after diagnosis as possible and without the added risk of delayed wound healing of irradiated skin. However, radiation field sizes are generally much larger, and cancer cells are more hypoxic due to disruption of blood supply during surgery.24 In the author’s practice, preoperative radiation therapy is preferred, primarily due to the smaller field size.
Figure 6.
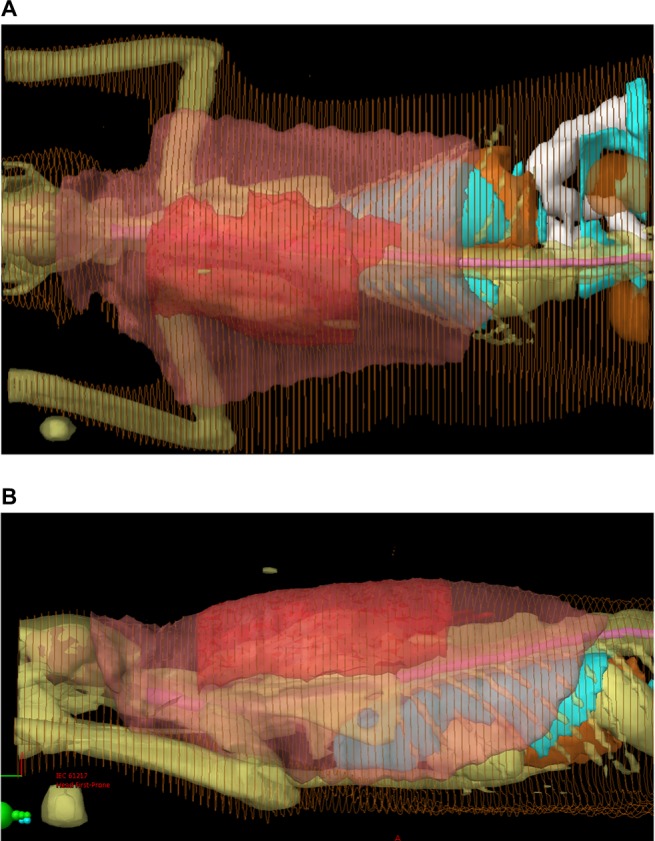
Radiation treatment plan for intensity modulated radiation therapy in a cat with FISS.
Notes: (A) Dorsal 3D image of radiation plan for an 8 cm interscapular FISS. (B) Lateral view 3D image of radiation plan for an 8 cm interscapular FISS. The darker red denotes the gross tumor volume (GTV). The lighter pink denotes the planned treatment volume, which is the GTV plus 4–5 cm of normal surrounding tissue. Photos courtesy of Dr Koichi Nagata.
Abbreviation: FISS, Feline injection site sarcomas.
With most definitive radiation protocols, radiation treatments are delivered Monday through Friday over the course of several weeks to a total treatment dose of 32–63 Gy.27,39–42,44 Side effects of radiation in cats are generally mild and primarily include dry desquamation of the skin; although as mentioned, delayed wound healing is possible. Computer-based radiation treatment plans and linear accelerators with on-board imaging capabilities are being utilized more frequently in the treatment of FISS to help minimize radiation damage to the surrounding normal structures such as the spinal cord, lungs, heart, and intra-abdominal organs. It must be noted that even with an aggressive multimodal treatment approach, local recurrence is still possible. Cronin et al27 reported a 79% local treatment failure in cats treated with definitive radiation therapy followed by surgery. Overall, however, median DFIs are prolonged when compared with surgery alone and range from 398 days to >1,000 days.27,39–42,44 Even with the addition of radiation therapy, the surgeon’s ability to attain wide microscopic tumor-free margins is an important predictor in the likelihood of and time to recurrence.27,39–41
Less is known about the role of chemotherapy in the treatment of FISS. In the author’s opinion, the relatively low metastatic rate argues against its use as a systemic therapy to prevent or slow the progression of distant spread. However, because FISS are extremely locally invasive with recurrences noted even in the face of aggressive radiation therapy and surgery protocols, some oncologists include chemotherapy in an attempt to further prolong the DFI. Carboplatin, doxorubicin, liposomal doxorubicin, cyclophosphamide, ifosfamide, and lomustine are among the chemotherapeutics reported in the treatment of FISS, with response rates ranging from 0% to >50%.41,44–48 Nonetheless, when used in a gross disease setting, few of the responses seen with chemotherapy are durable.45–48
Local immunotherapy has also been used as an adjuvant to surgical excision. Jourdier et al49 investigated a genetically attenuated vaccinia virus vector expressing human interleukin-2 (NYVAC human IL-2) and a recombinant canarypox virus vector expressing feline interleukin-2 (ALVAC feline IL-2) in cats with fibrosarcoma. With this approach, the vector virus enters the cat’s cells in the vicinity of injection; these cells then produce IL-2, inciting a local antitumor immune response. Local delivery of IL-2 reduces the patient risk of systemic toxicity seen with systemic IL-2 administration. In the Jourdier study specifically, cats were assigned to one of three groups: control, NYVAC human IL-2, and ALVAC feline IL-2. All cats underwent surgical excision and placement of iridium-based brachytherapy beads. Cats in the immunotherapy treatment groups were subsequently injected with NYVAC human IL-2 or ALVAC feline IL-2 around the surgical scars. Immunotherapy treatments were well-tolerated with only self-limiting local inflammation noted, and 1-year local recurrence rates of 61%, 39%, and 28% were seen in control cats versus those treated with NYVAC human IL-2 or ALVAC feline IL-2, respectively.49 A later study was performed by Jas et al50 to further investigate the safety and efficacy of the ALVAC IL-2 immunotherapy. Again, all cats in this study had their tumors excised and were then treated with iridium-based brachytherapy. Cats were randomized to one of the following groups: control, low-dose ALVAC IL-2, or high-dose ALVAC IL-2. No differences in DFI were seen between low-dose and high-dose-treated groups. A significant reduction in DFI was seen in treated cats versus controls. Median DFI in treated cats was not reached (>730 days), whereas median DFI in control cats was 287 days (P=0.046). Treatments were well tolerated and no differences in degree of toxicity were noted between low- and high-dose groups.50 In 2014, the ALVAC feline IL-2 immunotherapy was released in Europe as an adjuvant treatment to surgery to reduce the risk of local tumor recurrence in cats with fibrosarcoma (Merial SAS, Lyon, France). ALVAC feline IL-2 was subsequently conditionally licensed by the United States Department of Agriculture (USDA) in 2015 (Merial Inc., Duluth, GA, USA). Indications for treatment include cats with fibrosarcoma (2–5 cm diameter) with no evidence of nodal or distant metastasis. Additional investigation of the safety and efficacy of ALVAC IL-2 is ongoing.
Prognosis
Most prognostic factors that have been identified are associated with local tumor recurrence. In the author’s opinion, this is logical as local treatment failure leading to humane euthanasia appears to be the most common cause of death in cats with FISS. As the primary tumor gets larger and begins to stretch the overlying skin and invade underlying tissues, skin ulceration, pain, persistent bleeding, infection, and necrosis negatively affect the cat’s quality of life, as seen in Figure 5. One common predictor of local recurrence is completeness of the surgical excision (ie, clean margins). While this is especially true for cats treated with surgery alone,25,37 incomplete margins following multimodal treatment has also been associated with shorter DFI.27,39–41
Number of prior surgeries has also been inversely associated with time to local recurrence; as the number of surgical attempts increases, the local DFI typically decreases.37,39 This finding is in line with the principle that the first attempt at tumor removal is the most successful. Prior to the first excision, the surrounding anatomy is still relatively normal, and with each subsequent tumor recurrence and removal attempt, the amount of tissue for closure becomes less, making excision with clean margins impossible.51 Also in line with this finding are results from the study of Hershey et al,25 which determined that median time to first recurrence (TFR) was significantly longer for cats with tumors excised at a referral institution when compared with those excised at a general practice (median TFR was 274 days versus 66 days, respectively). This study,25 like the study of Phelps et al,36 concluded that a radical first excision is essential to achieve longer times to recurrence.
One study concluded that histological type was significantly associated with overall survival. Cats with fibrosarcoma or nerve sheath tumor had significantly longer survival times when compared with cats with malignant fibrous histiocytomas. Reported median survival times were 640 days versus 645 days versus 290 days, respectively.30
Histological grade, determined by mitotic index, percentage of necrosis, and degree of pleomorphism, has been associated with the likelihood of distant metastasis in dogs with soft tissue sarcomas,52 and a similar trend has been noted in cats. Cats with higher grade FISS appear more likely to develop metastasis, and one study further demonstrated that cats with metastasis had shorter overall survival times.26 Admittedly, FISS tend to be more mitotically active, have an increased percentage of necrosis, and a greater degree of pleomorphism,10 suggesting that the application of the canine soft tissue sarcoma grading scheme52 to FISS may result in a higher reporting of high-grade tumors.9 In the author’s opinion, further investigation is warranted to completely elucidate the role of histological grade in FISS management.
Prevention and vaccination guidelines
In 1996, the Vaccine-Associated Feline Sarcoma Task Force (VAFST) was formed with missions to propose changes in vaccination protocols to reduce the risk of FISS and to promote FISS research. In 1998, the American Association of Feline Practitioners (AAFP) developed a Feline Vaccination Advisory Panel to develop recommendations for appropriate vaccination protocols based on individual risk assessment. These groups, and others including the European Advisory Board on Cat Diseases (ABCD) and the World Small Animal Veterinary Association (WSAVA), recognize the value and importance of vaccines in the prevention of feline infectious diseases and have formulated recommendations to minimize risk of FISS development in cats. Their recommendations are also geared toward early detection and improved treatment success of FISS.13,33,53–58
One distinction that has been made is the difference between core and noncore vaccines. Core vaccines are recommended for all cats and include feline panleukopenia (FPV), feline herpesvirus-1 (FHV-1), feline calicivirus (FCV), and rabies, in endemic areas or where required by law. Non-core vaccines, including FeLV, FIV, Chlamydophila felis, Bordetella bronchiseptica, feline infectious peritonitis (FIP), and dermatophyte vaccines, should be given based on individual risk/benefit assessment. Risk variables to consider when formulating an individual cat’s vaccination protocol include age, lifestyle, and environment, and cats should only be vaccinated as often as necessary to maintain protection against infectious agents to which they have a realistic risk of exposure. Following these recommendations avoids unnecessary vaccination of cats.56,57
Administration of vaccines subcutaneously (versus intramuscularly) and at recommended vaccination sites has also been suggested. Subcutaneous administration allows for earlier, easier detection of any lumps that may develop postvaccination. Recommended vaccination sites include below the right elbow for FPV, FHV-1, and FCV vaccines, below the right stifle for rabies vaccines, and below the left stifle for FeLV vaccines. Alternatively, vaccination in the tail has been demonstrated as well-tolerated and efficacious.38 While adherence to these recommendations does not reduce the likelihood of FISS development, cats with tumors located distally on their limbs or tails may be excellent candidates for amputation and, therefore, complete surgical excision. Vaccination between the scapulae and even more proximally on the limbs is emphatically discouraged as complete surgical excision of FISS in these locations is nearly impossible.56–58
Because an association between chronic inflammation and FISS development has been suggested, the type of vaccine should also be considered. Admittedly, the role of adjuvants in the etiology of FISS remains unclear. However, because adjuvants are used to boost the immune reaction, some experts suggest avoidance of adjuvanted vaccines in cats.56–58
Finally, although FISS is a rare complication of vaccination in cats, it is important to inform owners of this risk so that they can closely monitor their cats following vaccine administration. Early detection is a key factor in treatment success. Accurate maintenance of individual patient’s medical records is also recommended. Information documented should include: 1) vaccine(s) recommended; 2) date of administration; 3) name of the person administering the vaccine; 4) vaccine name, lot or serial number, expiration date, and manufacturer; 5) site and route of administration; 6) concurrent medications and/or therapy; and 7) recommendations for future vaccinations. Currently, some vaccine manufacturers are willing to provide funds to offset the cost of treatment in cats with FISS. Excellent record keeping and documentation on the part of the veterinarian are undoubtedly useful in obtaining these funds.57
Footnotes
Disclosure
Dr Saba has been actively involved in clinical trials evaluating the safety and efficacy of ALVAC IL-2 in cats with fibrosarcoma in conjunction with Merial Inc., Duluth, GA, USA. The author reports no other conflicts of interest in this work.
References
- 1.Hendrick MJ, Dunagan CA. Focal necrotizing granulomatous panniculitis associated with subcutaneous injection of rabies vaccine in cats and dogs: 10 cases (1988–1989) J Am Vet Med Assoc. 1991;198(2):304–305. [PubMed] [Google Scholar]
- 2.Hendrick MJ, Goldschmidt MH. Do injection site reactions induce fibrosarcomas in cats? J Am Vet Med Assoc. 1991;199(8):968. [PubMed] [Google Scholar]
- 3.Hendrick MJ, Goldschmidt MH, Shofer FS, Wang YY, Somlyo AP. Post-vaccinal sarcomas in the cat: epidemiology and electron probe microanalytical identification of aluminum. Cancer Res. 1992;52(19):5391–5394. [PubMed] [Google Scholar]
- 4.Burton G, Mason KV. Do postvaccinal sarcomas occur in Australian cats? Aust Vet J. 1997;75(2):102–106. doi: 10.1111/j.1751-0813.1997.tb14167.x. [DOI] [PubMed] [Google Scholar]
- 5.Dean RS, Pfeiffer DU, Adams VJ. The incidence of feline injection site sarcomas in the United Kingdom. BMC Vet Res. 2013;9:17. doi: 10.1186/1746-6148-9-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martano M, Morello E, Ughetto M, et al. Surgery alone versus surgery and doxorubicin for the treatment of feline injection-site sarcomas: a report on 69 cases. Vet J. 2005;170(1):84–90. doi: 10.1016/j.tvjl.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 7.Hendrick MJ, Brooks JJ. Postvaccinal sarcomas in the cat: histology and immunohistochemistry. Vet Pathol. 1994;31(1):126–129. doi: 10.1177/030098589403100121. [DOI] [PubMed] [Google Scholar]
- 8.Hendrick MJ. Feline vaccine-associated sarcomas. Cancer Invest. 1999;17(4):273–277. doi: 10.3109/07357909909040597. [DOI] [PubMed] [Google Scholar]
- 9.Couto SS, Griffey SM, Duarte PC, Madewell BR. Feline vaccine-associated fibrosarcoma: morphologic distinctions. Vet Pathol. 2002;39(1):33–41. doi: 10.1354/vp.39-1-33. [DOI] [PubMed] [Google Scholar]
- 10.Doddy FD, Glickman LT, Glickman NW, Janovitz EB. Feline fibrosarcomas at vaccination sites and non-vaccination sites. J Comp Pathol. 1996;114(2):165–174. doi: 10.1016/s0021-9975(96)80005-3. [DOI] [PubMed] [Google Scholar]
- 11.Hendrick MJ, Shofer FS, Goldschmidt MH, et al. Comparison of fibrosarcomas that developed at vaccination sites and at nonvaccination sites in cats: 239 cases (1991–1992) J Am Vet Med Assoc. 1994;205(10):1425–1429. [PubMed] [Google Scholar]
- 12.Kass PH, Barnes WG, Jr, Spangler WL, Chomel BB, Culbertson MR. Epidemiologic evidence for a causal relation between vaccination and fibrosarcoma tumorigenesis in cats. J Am Vet Med Assoc. 1993;203(3):396–405. [PubMed] [Google Scholar]
- 13.Vaccine-Associated Feline Sarcoma Task Force The current understanding and management of vaccine-associated sarcomas in cats. J Am Vet Med Assoc. 2005;226(11):1821–1842. doi: 10.2460/javma.2005.226.1821. [DOI] [PubMed] [Google Scholar]
- 14.Kass PH, Spangler WL, Hendrick MJ, et al. Multicenter case-control study of risk factors associated with development of vaccine-associated sarcomas in cats. J Am Vet Med Assoc. 2003;223(9):1283–1292. doi: 10.2460/javma.2003.223.1283. [DOI] [PubMed] [Google Scholar]
- 15.Macy DW. Vaccine adjuvants. Semin Vet Med Surg (Small Anim) 1997;12(3):206–211. doi: 10.1016/s1096-2867(97)80034-5. [DOI] [PubMed] [Google Scholar]
- 16.Macy DW, Hendrick MJ. The potential role of inflammation in the development of postvaccinal sarcomas in cats. Vet Clin North Am Small Anim Pract. 1996;26(1):103–109. doi: 10.1016/s0195-5616(96)50009-4. [DOI] [PubMed] [Google Scholar]
- 17.Lester S, Clemett T, Burt A. Vaccine site-associated sarcomas in cats: clinical experience and a laboratory review (1982–1993) J Am Anim Hosp Assoc. 1996;32(2):91–95. doi: 10.5326/15473317-32-2-91. [DOI] [PubMed] [Google Scholar]
- 18.Gobar GM, Kass PH. World wide web-based survey of vaccination practices, postvaccinal reactions, and vaccine site-associated sarcomas in cats. J Am Vet Med Assoc. 2002;220(10):1477–1482. doi: 10.2460/javma.2002.220.1477. [DOI] [PubMed] [Google Scholar]
- 19.Srivastav A, Kass PH, McGill LD, Farver TB, Kent MS. Comparative vaccine-specific and other injectable-specific risks of injection-site sarcomas in cats. J Am Vet Med Assoc. 2012;241(5):595–602. doi: 10.2460/javma.241.5.595. [DOI] [PubMed] [Google Scholar]
- 20.Buracco P, Martano M, Morello E, Ratto A. Vaccine-associated-like fibrosarcoma at the site of a deep nonabsorbable suture in a cat. Vet J. 2002;163(1):105–107. doi: 10.1053/tvjl.2001.0617. [DOI] [PubMed] [Google Scholar]
- 21.Carminato A, Vascellari M, Marchioro W, Melchiotti E, Mutinelli F. Microchip-associated fibrosarcoma in a cat. Vet Dermatol. 2011;22(6):565–569. doi: 10.1111/j.1365-3164.2011.00975.x. [DOI] [PubMed] [Google Scholar]
- 22.Daly MK, Saba CF, Crochik SS, et al. Fibrosarcoma adjacent to the site of microchip implantation in a cat. J Feline Med Surg. 2008;10(2):202–205. doi: 10.1016/j.jfms.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McEntee MC, Page RL. Feline vaccine-associated sarcomas. J Vet Intern Med. 2001;15(3):176–182. doi: 10.1892/0891-6640(2001)015<0176:fvs>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 24.Seguin B. Feline injection site sarcomas. Vet Clin North Am Small Anim Pract. 2002;32(4):983–95. viii. doi: 10.1016/s0195-5616(02)00022-0. [DOI] [PubMed] [Google Scholar]
- 25.Hershey AE, Sorenmo KU, Hendrick MJ, Shofer FS, Vail DM. Prognosis for presumed feline vaccine-associated sarcoma after excision: 61 cases (1986–1996) J Am Vet Med Assoc. 2000;216(1):58–61. doi: 10.2460/javma.2000.216.58. [DOI] [PubMed] [Google Scholar]
- 26.Romanelli G, Marconato L, Olivero D, Massari F, Zini E. Analysis of prognostic factors associated with injection-site sarcomas in cats: 57 cases (2001–2007) J Am Vet Med Assoc. 2008;232(8):1193–1199. doi: 10.2460/javma.232.8.1193. [DOI] [PubMed] [Google Scholar]
- 27.Cronin K, Page RL, Spodnick G, et al. Radiation therapy and surgery for fibrosarcoma in 33 cats. Vet Radiol Ultrasound. 1998;39(1):51–56. doi: 10.1111/j.1740-8261.1998.tb00325.x. [DOI] [PubMed] [Google Scholar]
- 28.Hauck M. Feline injection site sarcomas. Vet Clin North Am Small Anim Pract. 2003;33(3):553–7. vii. doi: 10.1016/s0195-5616(03)00006-8. [DOI] [PubMed] [Google Scholar]
- 29.Esplin DG, McGill LD, Meininger AC, Wilson SR. Postvaccination sarcomas in cats. J Am Vet Med Assoc. 1993;202(8):1245–1247. [PubMed] [Google Scholar]
- 30.Dillon CJ, Mauldin GN, Baer KE. Outcome following surgical removal of nonvisceral soft tissue sarcomas in cats: 42 cases (1992–2000) J Am Vet Med Assoc. 2005;227(12):1955–1957. doi: 10.2460/javma.2005.227.1955. [DOI] [PubMed] [Google Scholar]
- 31.Shaw SC, Kent MS, Gordon IK, et al. Temporal changes in characteristics of injection-site sarcomas in cats: 392 cases (1990–2006) J Am Vet Med Assoc. 2009;234(3):376–380. doi: 10.2460/javma.234.3.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vaccine-Associated Feline Sarcoma Task Force guidelines. Diagnosis and treatment of suspected sarcomas. J Am Vet Med Assoc. 1999;214(12):1745. No authors listed. [PubMed] [Google Scholar]
- 33.Morrison WB, Starr RM. Vaccine-associated feline sarcomas. J Am Vet Med Assoc. 2001;218(5):697–702. doi: 10.2460/javma.2001.218.697. [DOI] [PubMed] [Google Scholar]
- 34.Rousset N, Holmes MA, Caine A, Dobson J, Herrtage ME. Clinical and low-field MRI characteristics of injection site sarcoma in 19 cats. Vet Radiol Ultrasound. 2013;54(6):623–629. doi: 10.1111/vru.12067. [DOI] [PubMed] [Google Scholar]
- 35.Kidney BA, Ellis JA, Haines DM, Jackson ML. Evaluation of formalin-fixed paraffin-embedded tissues obtained from vaccine site-associated sarcomas of cats for DNA of feline immunodeficiency virus. Am J Vet Res. 2000;61(9):1037–1041. doi: 10.2460/ajvr.2000.61.1037. [DOI] [PubMed] [Google Scholar]
- 36.Phelps HA, Kuntz CA, Milner RJ, Powers BE, Bacon NJ. Radical excision with five-centimeter margins for treatment of feline injection-site sarcomas: 91 cases (1998–2002) J Am Vet Med Assoc. 2011;239(1):97–106. doi: 10.2460/javma.239.1.97. [DOI] [PubMed] [Google Scholar]
- 37.Davidson EB, Gregory CR, Kass PH. Surgical excision of soft tissue fibrosarcomas in cats. Vet Surg. 1997;26(4):265–269. doi: 10.1111/j.1532-950x.1997.tb01497.x. [DOI] [PubMed] [Google Scholar]
- 38.Hendricks CG, Levy JK, Tucker SJ, et al. Tail vaccination in cats: a pilot study. J Feline Med Surg. 2014;16(4):275–280. doi: 10.1177/1098612X13505579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cohen M, Wright JC, Brawner WR, Smith AN, Henderson R, Behrend EN. Use of surgery and electron beam irradiation, with or without chemotherapy, for treatment of vaccine-associated sarcomas in cats: 78 cases (1996–2000) J Am Vet Med Assoc. 2001;219(11):1582–1589. doi: 10.2460/javma.2001.219.1582. [DOI] [PubMed] [Google Scholar]
- 40.Bregazzi VS, LaRue SM, McNiel E, et al. Treatment with a combination of doxorubicin, surgery, and radiation versus surgery and radiation alone for cats with vaccine-associated sarcomas: 25 cases (1995–2000) J Am Vet Med Assoc. 2001;218(4):547–550. doi: 10.2460/javma.2001.218.547. [DOI] [PubMed] [Google Scholar]
- 41.Kobayashi T, Hauck ML, Dodge R, et al. Preoperative radiotherapy for vaccine associated sarcoma in 92 cats. Vet Radiol Ultrasound. 2002;43(5):473–479. doi: 10.1111/j.1740-8261.2002.tb01036.x. [DOI] [PubMed] [Google Scholar]
- 42.Hahn KA, Endicott MM, King GK, Harris-King FD. Evaluation of radiotherapy alone or in combination with doxorubicin chemotherapy for the treatment of cats with incompletely excised soft tissue sarcomas: 71 cases (1989–1999) J Am Vet Med Assoc. 2007;231(5):742–745. doi: 10.2460/javma.231.5.742. [DOI] [PubMed] [Google Scholar]
- 43.McLeod DA, Thrall DE. The combination of surgery and radiation in the treatment of cancer. A review. Vet Surg. 1989;18(1):1–6. doi: 10.1111/j.1532-950x.1989.tb01034.x. [DOI] [PubMed] [Google Scholar]
- 44.Eckstein C, Guscetti F, Roos M, Martin de las Mulas J, Kaser-Hotz B, Rohrer Bley C. A retrospective analysis of radiation therapy for the treatment of feline vaccine-associated sarcoma. Vet Comp Oncol. 2009;7(1):54–68. doi: 10.1111/j.1476-5829.2008.00173.x. [DOI] [PubMed] [Google Scholar]
- 45.Barber LG, Sorenmo KU, Cronin KL, Shofer FS. Combined doxorubicin and cyclophosphamide chemotherapy for nonresectable feline fibrosarcoma. J Am Anim Hosp Assoc. 2000;36(5):416–421. doi: 10.5326/15473317-36-5-416. [DOI] [PubMed] [Google Scholar]
- 46.Saba CF, Vail DM, Thamm DH. Phase II clinical evaluation of lomustine chemotherapy for feline vaccine-associated sarcoma. Vet Comp Oncol. 2012;10(4):283–291. doi: 10.1111/j.1476-5829.2011.00295.x. [DOI] [PubMed] [Google Scholar]
- 47.Poirier VJ, Thamm DH, Kurzman ID, et al. Liposome-encapsulated doxorubicin (Doxil) and doxorubicin in the treatment of vaccine-associated sarcoma in cats. J Vet Intern Med. 2002;16(6):726–731. [PubMed] [Google Scholar]
- 48.Rassnick KM, Moore AS, Northrup NC, et al. Phase I trial and pharmacokinetic analysis of ifosfamide in cats with sarcomas. Am J Vet Res. 2006;67(3):510–516. doi: 10.2460/ajvr.67.3.510. [DOI] [PubMed] [Google Scholar]
- 49.Jourdier TM, Moste C, Bonnet MC, et al. Local immunotherapy of spontaneous feline fibrosarcomas using recombinant poxviruses expressing interleukin 2 (IL2) Gene Ther. 2003;10(26):2126–2132. doi: 10.1038/sj.gt.3302124. [DOI] [PubMed] [Google Scholar]
- 50.Jas D, Soyer C, De Fornel-Thibaud P, et al. Adjuvant immunotherapy of feline injection-site sarcomas with the recombinant canarypox virus expressing feline interleukine-2 evaluated in a controlled monocentric clinical trial when used in association with surgery and brachytherapy. Trials Vaccinol. 2015;4:1–8. [Google Scholar]
- 51.Farese JP, Withrow SJ. Surgical oncology. In: Withrow SJ, Vail DM, Page RL, editors. Small Animal Clinical Oncology. 5th ed. St. Louis: Elsevier; 2013. pp. 149–150. [Google Scholar]
- 52.Kuntz CA, Dernell WS, Powers BE, Devitt C, Straw RC, Withrow SJ. Prognostic factors for surgical treatment of soft-tissue sarcomas in dogs: 75 cases (1986-1996) J Am Vet Med Assoc. 1997;211(9):1147–1151. [PubMed] [Google Scholar]
- 53.Romatowski J. Recommendations of the vaccine-Associated Feline Sarcoma Task Force. J Am Vet Med Assoc. 1997;210(7):890. [PubMed] [Google Scholar]
- 54.Starr RM. Vaccine-Associated Feline Sarcoma Task Force: a new model for problem solving in veterinary medicine. J Am Vet Med Assoc. 1998;213(10):1428–1429. [PubMed] [Google Scholar]
- 55.Elston T, Rodan H, Flemming D, et al. 1998 report of the American Association of Feline Practitioners and Academy of Feline Medicine Advisory Panel on Feline Vaccines. J Am Vet Med Assoc. 1998;212(2):227–241. [PubMed] [Google Scholar]
- 56.Richards JR, Elston TH, Ford RB, et al. The 2006 American Association of Feline Practitioners Feline Vaccine Advisory Panel report. J Am Vet Med Assoc. 2006;229(9):1405–1441. doi: 10.2460/javma.229.9.1405. [DOI] [PubMed] [Google Scholar]
- 57.Scherk MA, Ford RB, Gaskell RM, et al. AAFP Feline Vaccination Advisory Panel Report. J Feline Med Surg. 2013;15(9):785–808. doi: 10.1177/1098612X13500429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hartmann K, Day MJ, Thiry E, et al. European Advisory Board on Cat Diseases Feline injection-site sarcoma: ABCD guidelines on prevention and management. J Feline Med Surg. 2015;17(7):606–613. doi: 10.1177/1098612X15588451. [DOI] [PMC free article] [PubMed] [Google Scholar]


