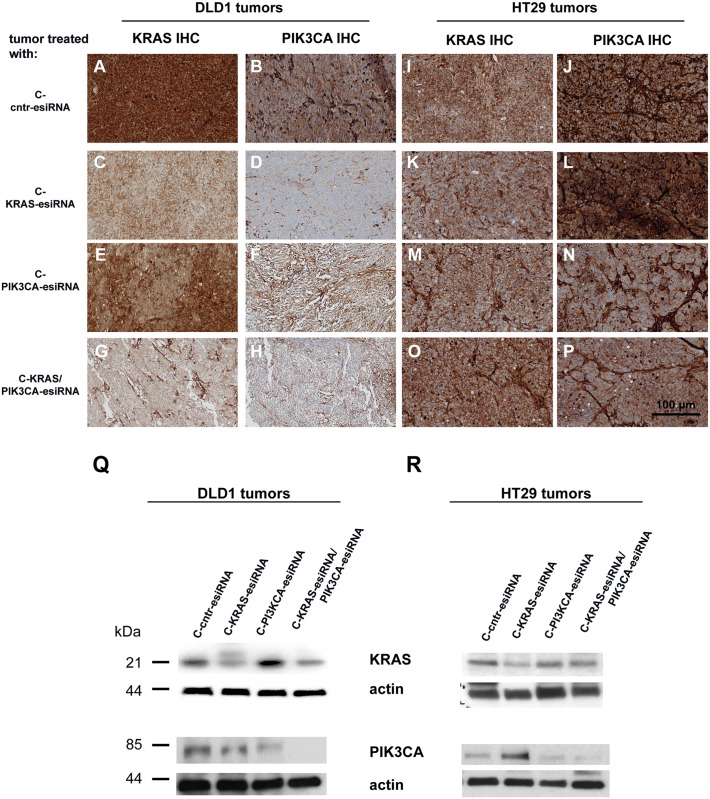
Fig 4. Treatment of CRC xenografts with cetuximab-protamine-siRNA markedly reduces siRNA target gene expression.
A-P. Paraffin sections from DLD1 tumors (A-H) and HT29 tumors (I-P) were prepared for immunohistochemical (IHC) analysis for siRNA targets KRAS and PIK3CA with antibody detecting KRAS (A, C, E, G and I, K, M, O) and PIK3CA (B, D, F, H and J, L, N, P) combined with suitable secondary antibodies and stained with diaminobenzidine (DAB) and hematoxylin and pictures taken at 20x magnification from regions without signs of necrosis. The application of KRAS siRNA coupled to cetuximab-sulfo-SMCC-protamine (C-KRAS-esiRNA) markedly reduced KRAS immunostaining (C) in DLD1 tumors, but not in HT29 (BRAF-mutated, PIK3CA-mutated; K) compared to control C-cntr-esiRNA (A, I). The treatment with C-PIK3CA-esiRNA reduced PIK3CA immunostaining in DLD1 (F) as well as HT29 tumors (N), as compared to control C-cntr-esiRNA in both cell lines (B, J). Interestingly, C-KRAS-esiRNA treatment also reduced PIK3CA staining in DLD1 tumors (D), but not in HT29 tumors (L). The combination treatment of tumors with KRAS- and PIK3CA-esiRNAs (C-KRAS/PIK3CA-esiRNA) resulted in even less KRAS and PIK3CA staining than the C-KRAS-esiRNA treatment in DLD1 tumors (G-H) and similar stainings of both in HT29 cells(O-P) as the C-PIK3CA-esiRNA monotherapy (M-N). Q-S. Western blots indicating siRNA target gene induced protein synthesis control in xenograft tumor tissue of cetuximab-protamine-esiRNA treated mice. Tumor tissue was processed for western blot as described, applied to SDS-PAGE, blotted and exposed for immunodetection by antibodies raised against KRAS, PIK3CA and actin as loading control. Application of cetuximab-protamine coupled to KRAS-esiRNA (C-KRAS-esiRNA) reduced KRAS protein levels in DLD1 (Q, top row), HT29 (R, top row) and SW480 (S, top row) tumor xenografts as compared to controls (actin row). In addition, C-KRAS-esiRNA treatment showed certain crosstalk to PI3K pathway signaling (third row from above) as indicated by reduced PIK3CA expression in DLD1 (Q), enhanced PIK3CA expression in HT29 (R) tumors and indifferent PIK3CA expression effect in SW480 (S) as compared to actin loading controls. C-PIK3CA-esiRNA treatment lead to reduced PIK3CA detection levels in all three xenograft tumor types (Q-S, third row from above) with even more pronounced suppression of PIK3CA protein by C-KRAS/PIK3CA-esiRNA combination in DLD1 and HT29 (Q-R, third row from above).