Scheme 3.
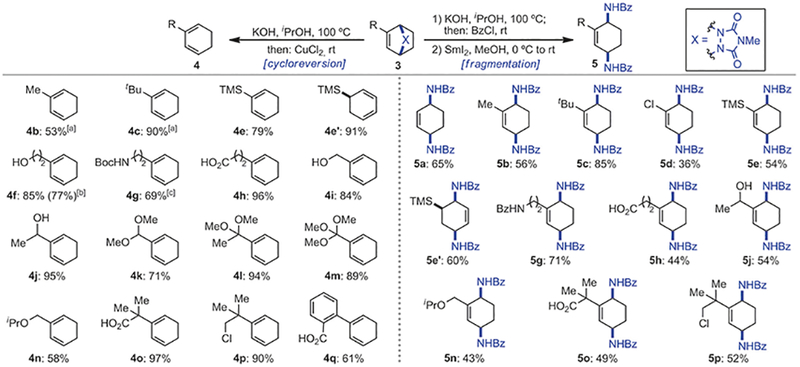
Cycloreversion and fragmentation of cycloadducts 3. Conditions for cycloreversion: 3 (0.4 mmol, 1.0 equiv), KOH (4.0 mmol, 10 equiv), iPrOH (0.2 m), 100°C, 12–24 h; then pH 7, CuCl2, (0.4 mmol, 1.0 equiv), 25°C, 1 min. Conditions for fragmentation: 1) 3 (0.2 mmol, 1.0 equiv), KOH (2.0 mmol, 10 equiv), iPrOH (0.3 m), 100°C, 12–24 h; then BzCl (1.0 mmol, 5.0 equiv), rt, 4–8 h. 2) Sml2 (0.5 mmol, 2.5 equiv), MeOH (0.1 m), 0°C to rt. Yields of isolated product after purification by flash chromatography. [a] Determined by ‘H NMR using internal standard. [b] Run on 3.15 mmol scale. [c] N2H4 was used instead of KOH followed by in situ protection with Boc2O.
