Scheme 3.
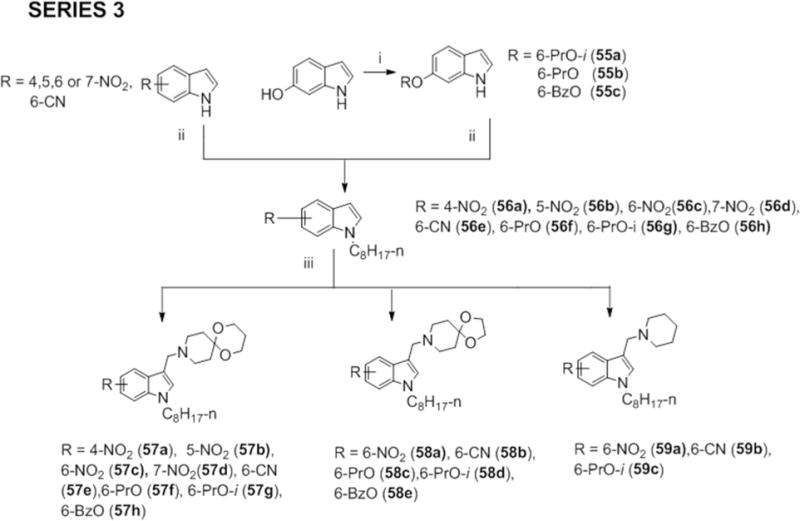
Reagents and conditions: (i) 1-iodopropane; 2-iodopropane; or benzyl bromide, K2CO3 or Cs2CO3, anhydrous DMF, 80°C, 3 – 4 h (ii) NaH, CH3(CH2)6CH2Br, anhydrous DMF, 0°C to RT, 2 – 8 h (iii) CH2O (aq., 36%), amines (1a, 12a or piperidine), CH3COOH, RT or ZnCl2, EtOH, RT, 3 – 24 h.
