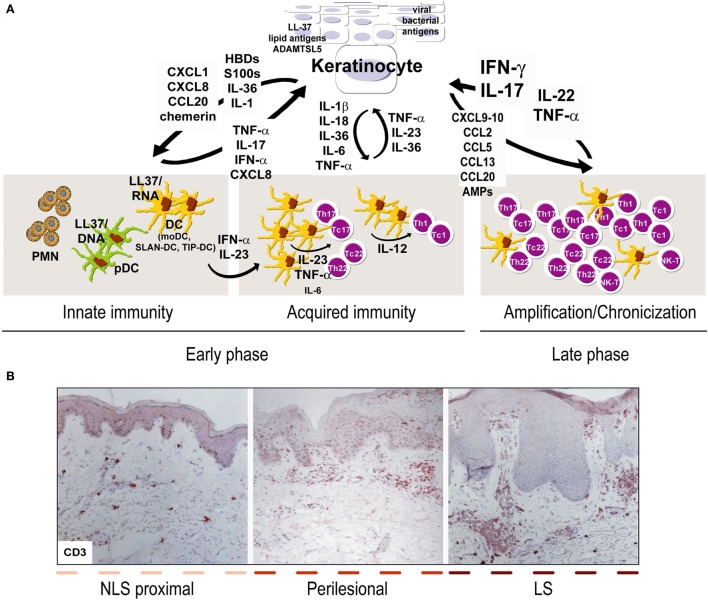
Figure 1.
Aberrant interplay of keratinocytes and immune cells in psoriasis. (A) Early upstream events in psoriasis include induction of innate immunity pathways and acquired responses, and keratinocytes represent the “key responding” skin cells by producing trigger factors, including LL37/nucleic acid complexes, lipid antigens, and ADAMTSL5, as well as pathogens of viral or bacterial origin. During this initial phase, keratinocytes also produce antimicrobial peptides (AMP), such as β-defensins (HBD) and S100 proteins, together with chemokines and cytokines of IL-1 family. Among AMP, LL37 activates plasmacytoid dendritic cells and myeloid DC (mDC), which routinely patrol uninvolved psoriatic skin, in particular, non-lesional (NLS) skin proximal to lesions, with consequent beginning of the adaptive immune phase. In early phase, SLAN-DC and TIP-DC, highly producing TNF-α, are also present. Hence, DC drive expansion of T lymphocytes, mostly Th17 and Th22 in the beginning and type-1 interferon (IFN)-γ-producing T cells, especially during the chronic phase. During acquired immunity phase, keratinocytes influence DC immune functions by producing cytokines derived from inflammasome pathway, and IL-36. T-cell infiltrate present during late/chronic phase of psoriasis establishes a cytokine milieu, mainly represented by IFN-γ, TNF-α, IL-17, IL-22, which dictates specific and pathogenic gene signatures in keratinocytes, which, thus, overexpress a number of inflammatory mediators. In parallel, under the influence of cytokines, in particular, IL-22 and IL-17, keratinocytes hyperproliferate and show altered differentiative programs. During the amplification/chronicization phase of the disease, unceasing cross-talk between keratinocytes and immune cells further amplifies inflammation and hyperplasia. (B) Both early and late phases of psoriasis can be found within the same psoriatic plaque, being it comprehensive of LS, perilesional and adjacent NLS areas, with markers of chronic inflammation (i.e., CD3+ T accumulation in the dermis) predominantly present in LS skin [CD3 stainings have been retrieved from Ref. (48)].