Figure 2. GFP‐Trap immunoprecipitation of caspase‐2 BiFC dimers identifies active caspase‐2‐interacting proteins.
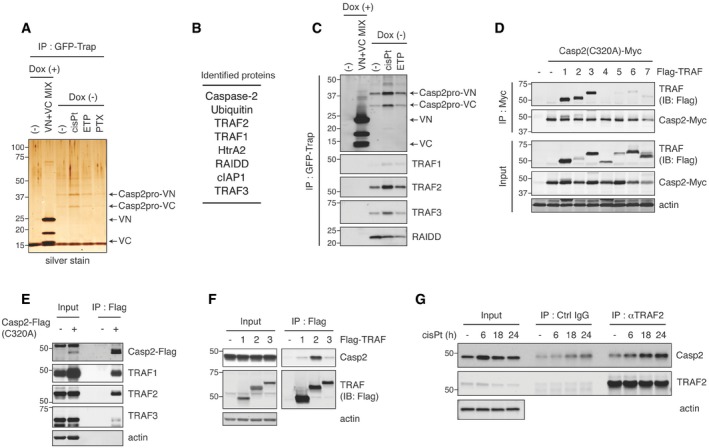
- Casp2pro BiFC cells were treated with mock, 20 μM cisplatin, 50 μM etoposide, or 100 nM paclitaxel for 24 h in the presence of 10 μM Q‐VD(OMe)‐OPh, followed by GFP‐Trap IP and silver stain to examine immunoprecipitates. For a negative control, Casp2pro BiFC expression was shut off by Dox and substituted with unconjugated VN‐VC BiFC dimers pooled from cells treated similarly to experimental samples. The procedure outline is in Fig EV2A.
- Casp2pro BiFC dimer‐interacting proteins identified by mass spectrometric analysis were listed in descending order of peptide abundance.
- Casp2pro BiFC cells were treated with or without 1 μg/ml Dox for 24 h, then mock, 20 μM cisplatin, or 50 μM etoposide for 24 h in the presence of 10 μM Q‐VD(OMe)‐OPh, followed by GFP‐Trap IP and IB. BiFC fragments were detected with anti‐GFP (FL, rabbit polyclonal antibody) IB.
- Casp2(C320A)‐Myc and Flag‐tagged TRAF1‐7 were co‐expressed in HEK293T cells for 48 h, followed by anti‐Myc IP and IB.
- Casp2(C320A)‐Flag was transfected into HeLa cells and expressed for 48 h, followed by anti‐Flag IP and IB.
- Flag‐TRAF1‐3 were transiently expressed in HeLa cells for 48 h followed by anti‐Flag IP and IB.
- HeLa cells were treated with 20 μM cisplatin in the presence of 10 μM Q‐VD(OMe)‐OPh for indicated periods. Lysates were prepared and immunoprecipitated with anti‐TRAF2 antibody or control IgG, followed by IB.
Source data are available online for this figure.
