Figure EV5. TRAF2 does not regulate removal of caspase‐2 ubiquitin modifications, which consist of both K48‐ and K63‐linked chains.
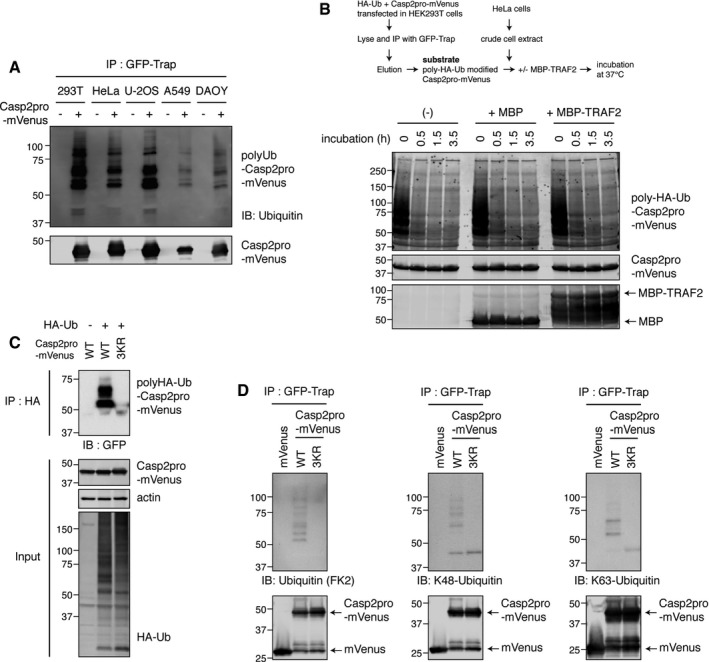
- HEK293T, HeLa, U‐2OS, A549, or DAOY cells were transfected with Casp2pro‐mVenus construct and cultured 48 h for expression. Casp2pro‐mVenus was immunoprecipitated by GFP‐Trap and blotted with anti‐ubiquitin antibody to detect ubiquitylated Casp2pro‐mVenus.
- In vitro deubiquitylation assay of caspase‐2. Casp2pro‐mVenus was ubiquitylated with HA‐ubiquitin in HEK293T cells and purified by GFP‐Trap IP and elution. Then, poly‐HA‐ubiquitin‐modified Casp2pro‐mVenus was added to HeLa cell lysate with or without recombinant MBP‐TRAF2 or MBP control protein. The mixture was incubated at 37°C for indicated periods and analyzed by immunoblot to assess whether TRAF2 could oppose caspase‐2 deubiquitylation.
- HA‐ubiquitin and Casp2pro‐mVenus (wild type or 3KR mutant) were co‐transfected into HEK293T cells, and lysates were immunoprecipitated by anti‐HA affinity beads and analyzed by IB.
- HEK293T cells were transfected with Casp2pro‐mVenus, wild type or 3KR mutant, followed by ubiquitylated Casp2pro‐mVenus purification as in (A). IB was carried out with anti‐ubiquitylated protein antibody (FK2), K48‐linkage‐specific, or K63‐linkage‐specific anti‐ubiquitin antibody.
Source data are available online for this figure.
