Abstract
Parasitic twin is a rare form of conjoined twins with an incidence ranging from 1 in 50,000 to 1,00,000 live births. In thoracopagus type, both hearts are joined together and often are associated with underlying congenital cardiac malformations. The separation surgery is a challenging task for both the surgeon as well as anesthetist due to the complexity of the procedure and long duration of surgery, carrying mortality close to 100% in case of significant cardiac fusion. Here, we are sharing anesthetic management of successful separation of a rare type of parasitic male conjoined twins who had connected hearts and common liver.
Keywords: Anesthesia, parasitic twins, surgery, thoracopagus
Introduction
Conjoined twins are a rare form of twin gestation resulting from incomplete division of zygote between 13th and 15th day after fertilization. Its incidence ranges from 1 in 50,000 to 1 in 100,000 live births with an overall survival rate of 25%.[1] The separation of conjoined twins is a challenging task for both the surgeon as well as anesthetist due to the complexity of the procedure and long duration of surgery. It becomes more challenging to maintain hemodynamic stability if both the hearts of the twins are joined together, carrying mortality close to 100% in case of significant cardiac fusion.[2]
Nowadays, twin surgeries are being increasingly done worldwide, but each case has its own specific difficulties requiring individualized care.[3] From anesthetist's point of view, here we are sharing our experience of a successful separation surgery underwent by a rare type of parasitic male conjoined twins who had joined hearts and common liver.
Case Report
A pregnant woman was brought to our emergency in labor pains and delivered a pair of male conjoined twins through normal vaginal delivery. Twins were joined at chest with a total weight of 3600 g. The large baby was morphologically normal while in small baby lower limbs were absent, upper limbs were rudimentary, and mouth opening was also absent [Figure 1]. At birth large baby cried after tactile stimulation but small one had only cardiac activity with no respiratory movements. At 5 min after birth large baby's Apgar score was 9/10 and smaller one had pink color, heart rate more than 100/min with movements of upper limbs. Twins were shifted to neonatal intensive care unit for further management.
Figure 1.
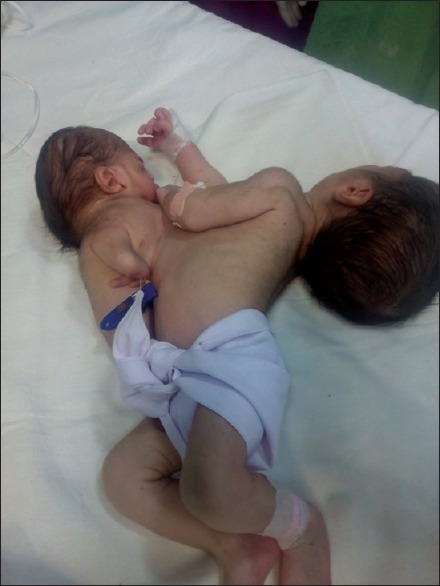
Before surgery
For the evaluation of extent of shared organ system and to find out associated congenital abnormalities, contrast-enhanced computerized tomography was done which revealed normal four-chambered heart and major vessels of large baby with short communicating channel between left ventricle of large baby and heart of small baby measuring 5 mm in diameter and 1 cm in length and also a communication between internal mammary arteries of both the babies. In abdomen, there was one liver shared between two babies, two-third volume in large baby and one-third in small baby. Furthermore, there was one large venous channel traversing the liver between inferior vena cava of large baby and rudimentary inferior vena cava/heart of both babies. Lungs, brain, pancreas, intestine, and gallbladder were normal in large baby. In small baby lungs, trachea, normal configuration of aorta, air in intestine, spleen, kidneys, and urinary bladder were not visualized. Upper gastrointestinal contrast study revealed no connection between intestine of two twins. Hematological and biochemical tests were within normal limits. In view of above finding, the small baby was diagnosed as nonsalvageable and was considered a parasite, so early separation was planned to save the large baby after obtaining parental consent.
After thorough preoperative evaluation, and discussion among members of multi-disciplinary team baby was shifted to warm operation theater on day 10 of life. Two multipara monitors were attached to upper limbs of both the babies. Hear rate in both the twins were same at 148/min and blood pressure of large baby was 86/68 mmHg. Respiratory rate of the large baby was 58/min and could not be appreciated in small baby. Two peripheral lines with 24 G cannulae were secured one each in right upper limb and lower limb of healthy baby. For beat-to-beat blood pressure monitoring, arterial line was secured with 24G cannula in radial artery of healthy baby. Injection atropine 0.1 mg was injected intravenously in large baby to check the cross circulation. The heart rate of parasitic twin also increased by 26/min confirming the cross circulation. Hence, we decided to induce healthy baby according to total body weight. For this, we preoxygenated healthy baby with Jackson Rees modification of Ayre's T-piece, and induction was achieved with sevoflurane along with 10 μgm injection fentanyl and 5 mg injection ketamine intravenously, and muscle relaxation was achieved by 7 mg injection succinylcholine intravenously. As both the babies were joined at thorax, the parasitic baby was lifted over the healthy baby to achieve supine position, and head of parasitic baby was extended to ease the intubation of healthy baby. The intubation was done through oral route with 3.5 mm internal diameter endotracheal tube and was fixed at 9 cm. The anesthesia was maintained with sevoflurane, atracurium, fentanyl, and blended oxygen. End-tidal CO2, temperature via nasopharyngeal catheter and urine output were also monitored.
Surgical incision was given at the junction of the twins. First of all, connecting channel between the ventricles of both the twins was ligated and separated. Then liver was separated from the parasitic twin and was repositioned into the healthy baby. The common vessels were ligated and separated. Ligation of sharing vessels and separation of hearts led to steep fall in saturation followed by nonrecordable vitals in parasitic baby.
Intraoperatively, there was fall in oxygen saturation whenever the parasitic baby came over the healthy baby which was improved by lifting up the parasitic baby. Furthermore, handling of the heart and liver led to episodes of hypotension and bradycardia for which surgery was temporarily was put on hold multiple times and finally, adrenaline and noradrenaline infusions were started to maintain blood pressure. Arterial blood gas analysis performed one hourly remained within normal limits. The whole surgery lasted for 3 h, and during this period 150 ml ringer lactate and 50 ml packed cell were infused.
After the completion of surgery [Figure 2], the baby was shifted to NICU with endotracheal tube in situ and adrenaline and noradrenaline infusion. Inotropes were tapered, and the baby was extubated on the 2nd day of surgery and was discharged on the 15th postoperative day on full breastfeed.
Figure 2.
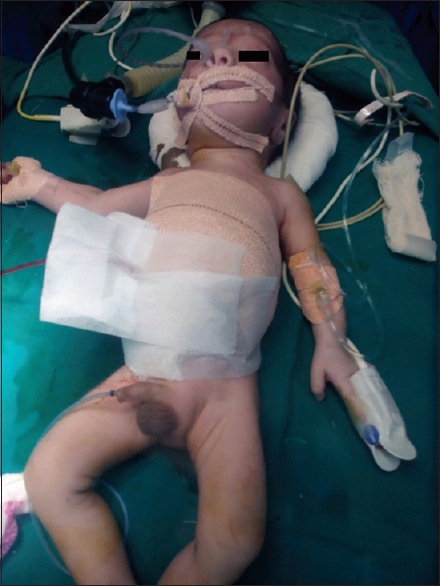
After surgery
Discussion
Prenatal diagnosis of conjoined twins has been made as early as 10 weeks of gestation.[1] The lack of prenatal information and spontaneous delivery always results in perinatal trauma in at least one of the twins worsening the surgical outcome.[3] In our case, the mother was admitted in active labor with no antenatal ultrasound record, and underwent normal vaginal delivery. Twinning was revealed at birth only, and fortunately well-formed baby escaped from the major perinatal insult.
In thoracopagus twins, both hearts are joined together and often are associated with underlying congenital cardiac malformations decreasing the overall survival rate.[4] Our case also had several episodes of bradycardia and hypotension whenever heart was handled, but every time, it was managed successfully, probably due to structurally normal heart.
In asymmetric conjoined twins, the partially developed twin is labeled as parasite as it derives its nutrition mainly from completely developed counterpart known as orthosite.[5,6] Twinning is three times more common in females in comparison to males.[1] Asymmetric/parasitic male twins, as in the present case, are rare.
Surgery should better be delayed till 6–12 months of age, as physical maturity is associated with better outcome. Surgeries done in neonatal period is very risky carrying a mortality rate of 50%.[7] However, in our case, one of the twins was parasite over the healthy one with connecting hearts and common liver; so as to save the healthy child we planned early separation.
Endotracheal intubation of thoraco-omphalopagus conjoined twins requires special positioning; we elevated one twin over another to facilitate intubation, this maneuver has been used previously also.[3] Before induction, it is necessary to check the cross-circulation so that fluid and drugs could be given accordingly. Like in our case, cross circulation was present which was assessed by clinical of effects of atropine.[8]
Separation surgery in twins requires meticulous intra-operative monitoring.[8] In our case, intra-arterial blood pressure monitoring helped us in timely detection and management of hemodynamic instability during traction over the heart and liver. Similarly, temperature should be monitored regularly to prevent hypothermia, especially in prolonged surgeries.[9] We used heating mattress, fluid warmer and radiant warmer to maintain normothermia. Central venous pressure should also be monitored to guide fluid therapy. Blood products should be prearranged anticipating massive blood loss.[5,8] Fortunately, in our case, blood loss was minimal requiring 50 ml of packed cell.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Osmanağaoğlu MA, Aran T, Güven S, Kart C, Ozdemir O, Bozkaya H, et al. Thoracopagus conjoined twins: A case report. ISRN Obstet Gynecol. 2011;2011:238360. doi: 10.5402/2011/238360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seo M, Chung IS, Karm MH, Oh JM, Shin WJ. Anesthetic management for separation of thoracopagus twins with complex congenital heart disease: A case report. Korean J Anesthesiol. 2015;68:295–9. doi: 10.4097/kjae.2015.68.3.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kobylarz K. Anaesthesia of conjoined twins – Case series. Anaesthesiol Intensive Ther. 2014;46:65–77. doi: 10.5603/AIT.2014.0014. [DOI] [PubMed] [Google Scholar]
- 4.Sharma G, Mobin SS, Lypka M, Urata M. Heteropagus (parasitic) twins: A review. J Pediatr Surg. 2010;45:2454–63. doi: 10.1016/j.jpedsurg.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 5.Kiran S, Kaur KP, Rattan KN, Rattan SK. Anaesthetic management of a patient with thoracopagus. S Afr J Anaesthesiol Analg. 2010;16:33–6. [Google Scholar]
- 6.Curry EK, Schraibman V. Epigastric heteropagus twinning. J Pediatr Surg. 2001;36:11–5. doi: 10.1053/jpsu.2001.24774. [DOI] [PubMed] [Google Scholar]
- 7.Chalam KS. Anaesthetic management of conjoined twins’ separation surgery. Indian J Anaesth. 2009;53:294–301. [PMC free article] [PubMed] [Google Scholar]
- 8.Kaniyil S, Pavithran P, Mubarak KK, Mohamed T. Anaesthetic challenges in conjoined twins’ separation surgery. Indian J Anaesth. 2016;60:852–5. doi: 10.4103/0019-5049.193685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lalwani J, Dubey K, Shah P. Anaesthesia for the separation of conjoined twins. Indian J Anaesth. 2011;55:177–80. doi: 10.4103/0019-5049.79902. [DOI] [PMC free article] [PubMed] [Google Scholar]


