Figure 2. UBLs of HOIL-1L and SHARPIN Bind to HOIP UBA in a Highly Cooperative Manner.
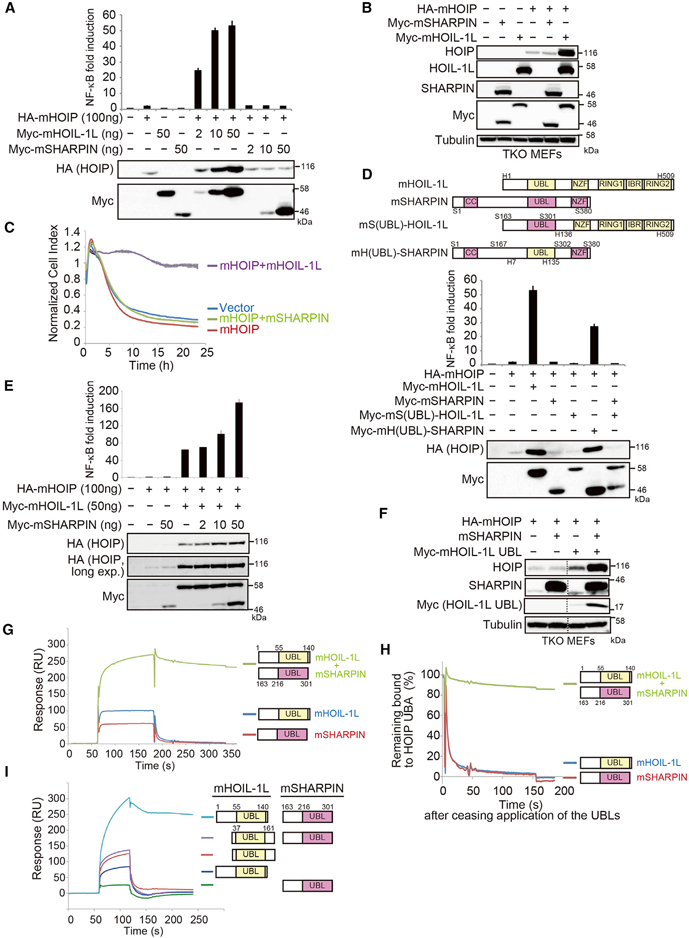
(A) The indicated expression plasmids and 5× NF-κB luciferase reporters were transfected into HEK293T cells. Cell lysates were probed with indicated antibodies, and NF-κB activity was measured by luciferase assays (means ± SEM, n = 3).
(B) Cell lysates of TKO MEFs reconstituted with the indicated proteins were probed as indicated.
(C) TKO MEFs expressing the indicated proteins were stimulated with TNF-α (10 ng/ml), and cell viability was measured on a RTCA (mean ± SEM, n = 3).
(D) Schematic representation of HOIL-1L, SHARPIN, and their mutants. The indicated expression plasmids and 5× NF-κB luciferase reporters were transfected and analyzed as described in Figure 2A (means ± SEM, n = 3).
(E) The indicated plasmids and 5× NF-κB luciferase reporter were transfected into HEK293T cells and analyzed as described in Figure 2A (mean ± SEM, n = 3).
(F) Cell lysates of TKO MEFs reconstituted with the indicated proteins were probed as indicated. Vertical dashed lines indicate cropped blots.
(G) Glutathione S-transferase (GST)-mHOIP466–630 was immobilized on an SPR sensor chip via a GST antibody (Figures 2G and 2I). mHOIL-1L1–140 alone (500 μg/ml), mSHARPIN163–301 alone (500 mg/ml), or both proteins together (500 mg/ml each) were used as analytes.
(H) Plots of data in Figure 2G, with responses normalized against the value at the time application of UBL ceased.
(I) Interactions between UBLs containing or lacking the N-terminal region were analyzed as described in Figure 2G.
