Figure 4. Novel HOIL-1L-SHARPIN Interactions Mediated by LTMs.
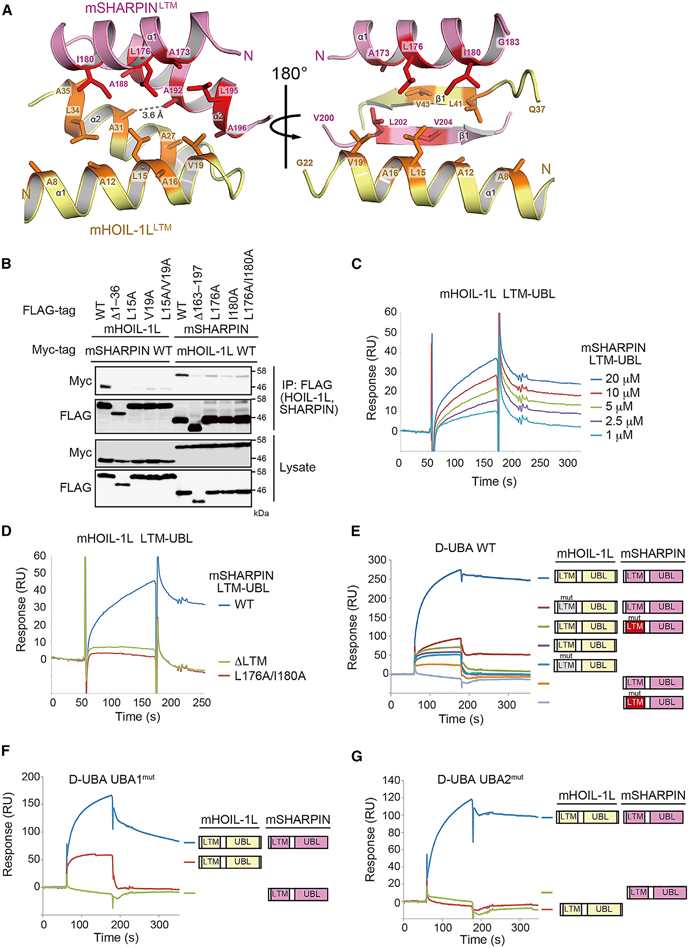
(A) Heterodimeric interface ofTD formed by LTMsofmHOIL-ILand mSHARPIN. The residuesforming the hydrophobiccore of TD areshown asstick models and highlighted in red (mSHARPIN) or orange (mHOIL-1L). β1 strands and α2 helices of both molecules are eliminated from the ribbon models in the left and right panels, respectively.
(B) The indicated expression plasmidsweretransfected into HEK293T HOIP KO cells. Cell lysates and anti-FLAG immunoprecipitateswere probed as indicated.
(C) mHOIL-1L1–189-strepwas immobilized on a SPR sensorchip with anti-strep antibody. Binding between mHOIL-1L1–189 and MBP-mSHARPIN UBL163–301 was evaluated.
(D) Interactions between mHOIL-1L1–189 and MBP-mSHARPIN mutants were analyzed as described in Figure 4C. mSHARPIN163–301 WT, L176A/I180A, and mSHARPIN198–318 ΔLTM (40 mM) were used as analytes.
(E-G) GST-mHOIP D-UBA (aa 466–630) WT (E), UBA1mut (F), and UBA2mut (G) were immobilized on a SPR sensor chip with anti-GST antibody. Binding to UBLs of WT or LTMmut was analyzed like in Figure 2G.
