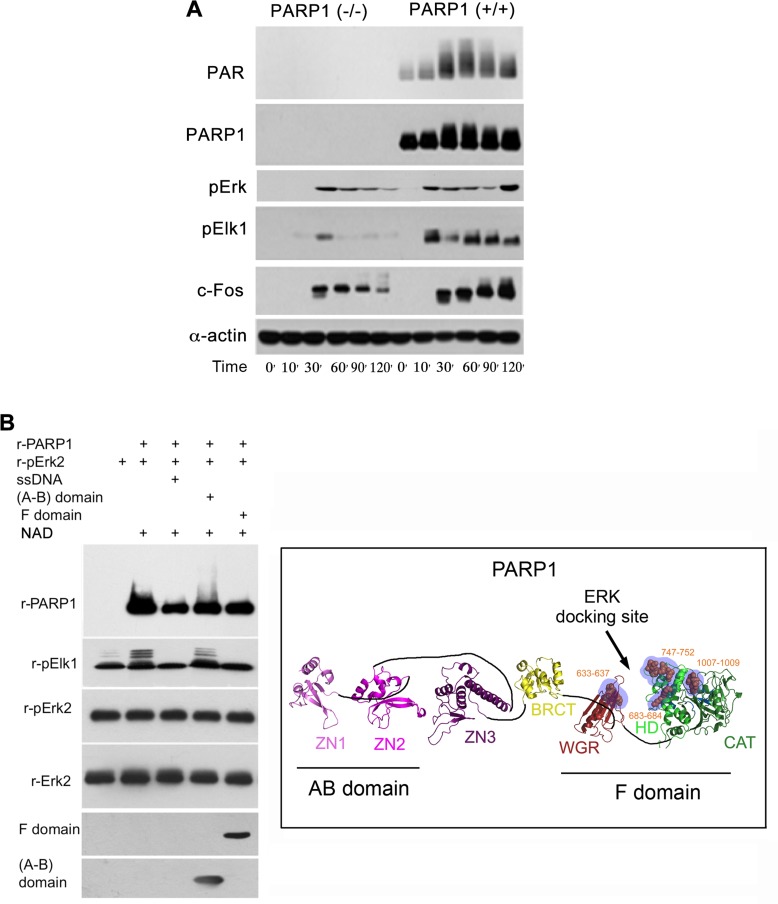
Figure 2. A PARP1 dependent Erk-activity in the nucleus.
(A) Immunolabeled phosphorylated Erk1/2, and transcription factors Elk1 and c-Fos monitored during 120 min following treatment with PMA (200 nM, 20 min) in the nuclear and cytoplasmic protein extracts of MEF and PARP1-KO MEF. Representative results of 3 different experiments are displayed. (B) Right: A PARP1 dependent Erk activity in a cell-free system. Immunolabeled recombinant PARP1 (100 ng/sample) polyADP-ribosylated in the presence of NAD (10 µM), ATP (1 mM) and recombinant phosphorylated Erk2 (100 ng/sample). Recombinant Elk1 (600 ng/sample) was phosphorylated in this system by recombinant phosphorylated Erk2. PARP1 polyADP-ribosylation was attenuated in the presence of recombinant F domain of PARP1 carrying its Erk binding sites (aa556-1014 domain; 100 ng/sample), or in the presence of nicked DNA (ssDNA;1 mg/sample). The recombinant AB domain of PARP1 which includes its DNA binding sites (1–20 aa domain, 100 ng/sample) did not affect Erk-induced PARP1 activation. Representative results of 3 different experiments are displayed. Left: A ribbon structural model for the open conformation of PARP1 with consensus docking sites for phosphorylated Erk (orange spheres in blue shaded region) in its catalytic (CAT), helical (HD) and WGR domains (From: Figure 6A in ref #7).