Figure 3. TopBP1 BRCT5 may bind BLM and 53BP1 through similar mechanisms.
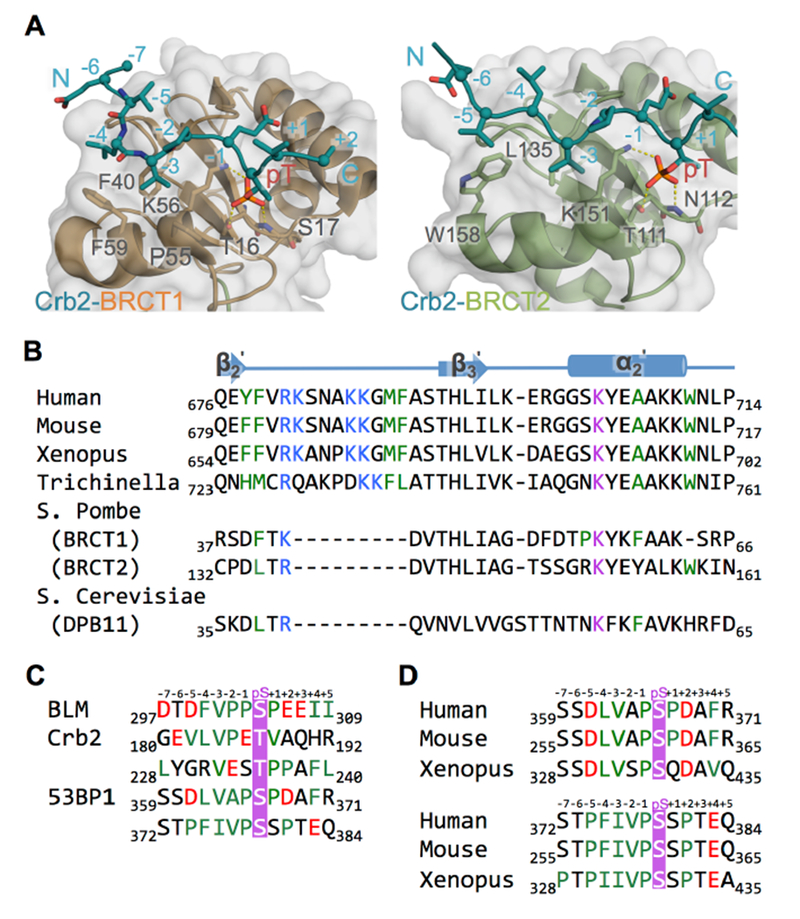
A. Structures of Rad4TopBP1 BRCT repeats bound to Crb253BP1. Rad4 BRCT1/Crb2 (left) and Rad4 BRCT2/Crb2 (right) structures (PDB code: 4BU0) are aligned with the TopBP1/BLM complex as in Figure 2A. Both structures have surface and cartoon displayed for Rad4TopBP1 (BRCT1 in orange, BRCT2 in green) and only cartoon displayed for Crb253BP1 (blue). Interacting residues are displayed as sticks. Hydrogen bonds are indicated by yellow dash-lines.
B. Sequence alignment of the peptide binding region TopBP1 BRCT5 homologues. Residues from the positively charged β2’-β3’ loop are colored blue, residues from phosphate binding pocket are colored purple, and residues lining hydrophobic groove are colored green.
C. Sequence alignment of BLM, Crb2 and 53BP1 phosphopeptide partners for TopBP1 BRCT5. Phosphorylated residues are colored purple, negatively charged residues are colored red and hydrophobic residues are colored green.
D. Sequence alignment of potential TopBP1 BRCT5 binding regions in 53BP1 homologues. Coloring is as in Figure 3C.
