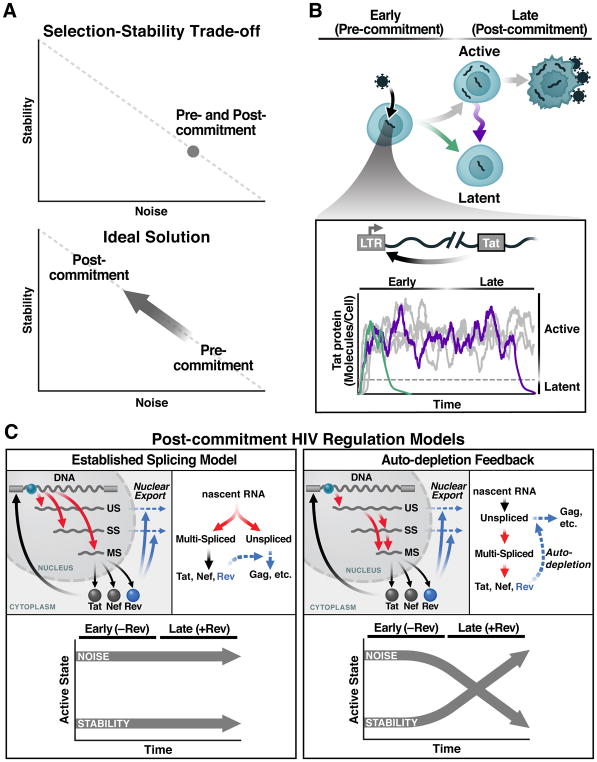
Figure 1. The Selection-Stability Tradeoff in Noise-Driven Fate Regulation and the HIV-Specific Case.
(A) Regulatory circuits that amplify fluctuations in gene-expression (noise; e.g., as measured by σ2/μ2) enable probabilistic fate selection but at the cost of reduced stability of the selected fate, whereas low-noise circuits are stable but have limited capacity for probabilistic fate selection (upper panel). The ideal scheme for enabling both probabilistic fate selection and stability (i.e., commitment) to the selected state is for a fate circuit to exhibit high noise during early times but subsequently attenuate noise once a fate has been selected (lower panel).
(B) HIV’s fate decision in CD4+ T cells: early after infection, infected cells commit to either latent or active infection with actively infected cells producing virus after a delay of ~1 day and then dying after ~2 days. Inset: the HIV Tat positive-feedback circuit regulates the active and latent states with Tat transactivation of the LTR promoter (HIV’s only promoter) being obligate for active replication and low Tat levels enabling viral latency. As long as positive feedback persists, it generates large fluctuations in gene expression, enabling entry to latency (green) but potentially destabilizing the active state (purple).
(C) Two models of HIV gene regulation during active replication. Left: The established model of co-transcriptional RNA alternative splicing; US, SS, and MS RNAs are independently generated and thus Rev-mediated export of US and SS does not deplete MS RNA. Right: The precursor auto-depletion model relies on a serial cascade of post-transcriptional splicing where US and SS RNAs are precursors for MS RNA and Rev-mediated export of US and SS precursors depletes MS RNA establishing negative feedback. Lower panels indicate noise and stability dynamics for each model.