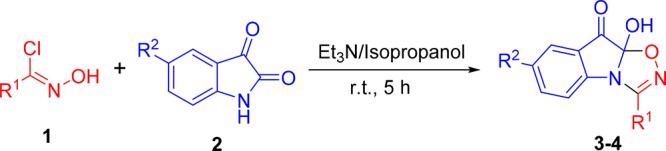
Table 2. Select Examples of [3 + 2] Cycloaddition of in Situ-Generated Nitrile Oxides and Indoline-2,3-dionea.
| entry | 1 (R1) | 2 (R2) | 3 | yieldb (%) |
|---|---|---|---|---|
| 1 | 1a (Ph) | 2a (H) | 3a | 84 |
| 2 | 1b (4-F C6H4) | 2a (H) | 3b | 88 |
| 3 | 1c (4-OCH3 C6H4) | 2a (H) | 3c | 70 |
| 4 | 1d (i-Pr) | 2a (H) | 3d | 46 |
| 5 | 1e (4-CH3 C6H4) | 2a (H) | 3e | 77 |
| 6 | 1f (3-CH3 C6H4) | 2a (H) | 3f | 75 |
| 7 | 1g (2-CH3 C6H4) | 2a (H) | 3g | 79 |
| 8 | 1h (4-Et C6H4) | 2a (H) | 3h | 76 |
| 9 | 1i (3-F C6H4) | 2a (H) | 3i | 88 |
| 10 | 1j (2-F C6H4) | 2a (H) | 3j | 86 |
| 11 | 1k (4-Cl C6H4) | 2a (H) | 3k | 85 |
| 12 | 1l (3-Cl C6H4) | 2a (H) | 3l | 83 |
| 13 | 1m (2-Cl C6H4) | 2a (H) | 3m | 89 |
| 14 | 1n (4-Br C6H4) | 2a (H) | 3n | 82 |
| 15 | 1o (C4H3S)c | 2a (H) | 3o | 64 |
| 16 | 1a (Ph) | 2b (F) | 3p | 72 |
| 17 | 1e (4-CH3 C6H4) | 2b (F) | 3q | 69 |
| 18 | 1c (4-OCH3 C6H4) | 2b (F) | 3r | 70 |
| 19 | 1b (4-F C6H4) | 2b (F) | 3s | 87 |
| 20 | 1a (Ph) | 2c (OCH3) | 3t | 87 |
| 21 | 1e (4-CH3 C6H4) | 2c (OCH3) | 3u | 84 |
| 22 | 1g (2-CH3 C6H4) | 2c (OCH3) | 3v | 78 |
| 23 | 1b (4-F C6H4) | 2c (OCH3) | 3w | 96 |
| 24 | 1j (2-F C6H4) | 2c (OCH3) | 3x | 85 |
| 25 | 1a (Ph) | 2d (CH3) | 3y | 85 |
| 26 | 1e (4-CH3 C6H4) | 2d (CH3) | 3z | 82 |
| 27 | 1g (2-CH3 C6H4) | 2d (CH3) | 4a | 81 |
| 28 | 1b (4-F C6H4) | 2d (CH3) | 4b | 92 |
| 29 | 1i (3-F C6H4) | 2d (CH3) | 4c | 89 |
| 30 | 1j (2-F C6H4) | 2d (CH3) | 4d | 88 |
| 31 | 1k (4-Cl C6H4) | 2d (CH3) | 4e | 80 |
| 32 | 1a (Ph) | 2e (Br) | 4f | 83 |
| 33 | 1e (4-CH3 C6H4) | 2e (Br) | 4g | 81 |
| 34 | 1g (2-CH3 C6H4) | 2e (Br) | 4h | 82 |
| 35 | 1b (4-F C6H4) | 2e (Br) | 4i | 87 |
| 36 | 1k (4-Cl C6H4) | 2e (Br) | 4j | 85 |
General conditions: hydroxybenzimidoyl chloride (1, 24 mmol, 1.2 equiv), indoline-2,3-dione (2, 20 mmol), base (40 mmol, 2 equiv), and isopropanol (50 mL).
Isolated yield based on 2.
C4H3S = thiophene.
