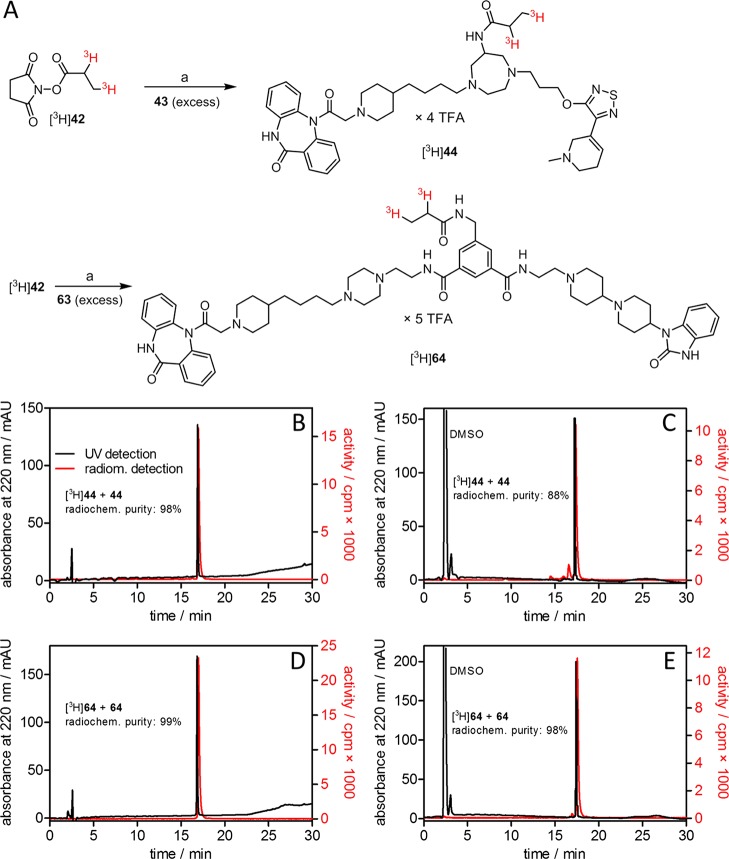
Figure 4.
Preparation, purity, and identity control of the radiolabeled dibenzodiazepinone derivatives [3H]44 and [3H]64. (A) Synthesis of [3H]44 and [3H]64 by [3H]propionylation of amine precursors 43 and 63, respectively, using succinimidyl [3H]propionate ([3H]42). Reagents and conditions: (a) DIPEA, DMF, rt, 1.5 h, radiochemical yields: 36% ([3H]44) and 35% ([3H]64). (B,C) HPLC analysis of [3H]44 (0.18 μM) spiked with “cold” 44 (3 μM), analyzed 3 days after synthesis (B) and after 10 months of storage at −20 °C in EtOH/H2O (1:1) (C). (D,E) HPLC analysis of [3H]64 (0.23 μM) spiked with “cold” 64 (3 μM), analyzed 3 days after synthesis (D) and after 10 months of storage at −20 °C in EtOH/H2O (1:1) (E). HPLC conditions are provided in the Supporting Information.