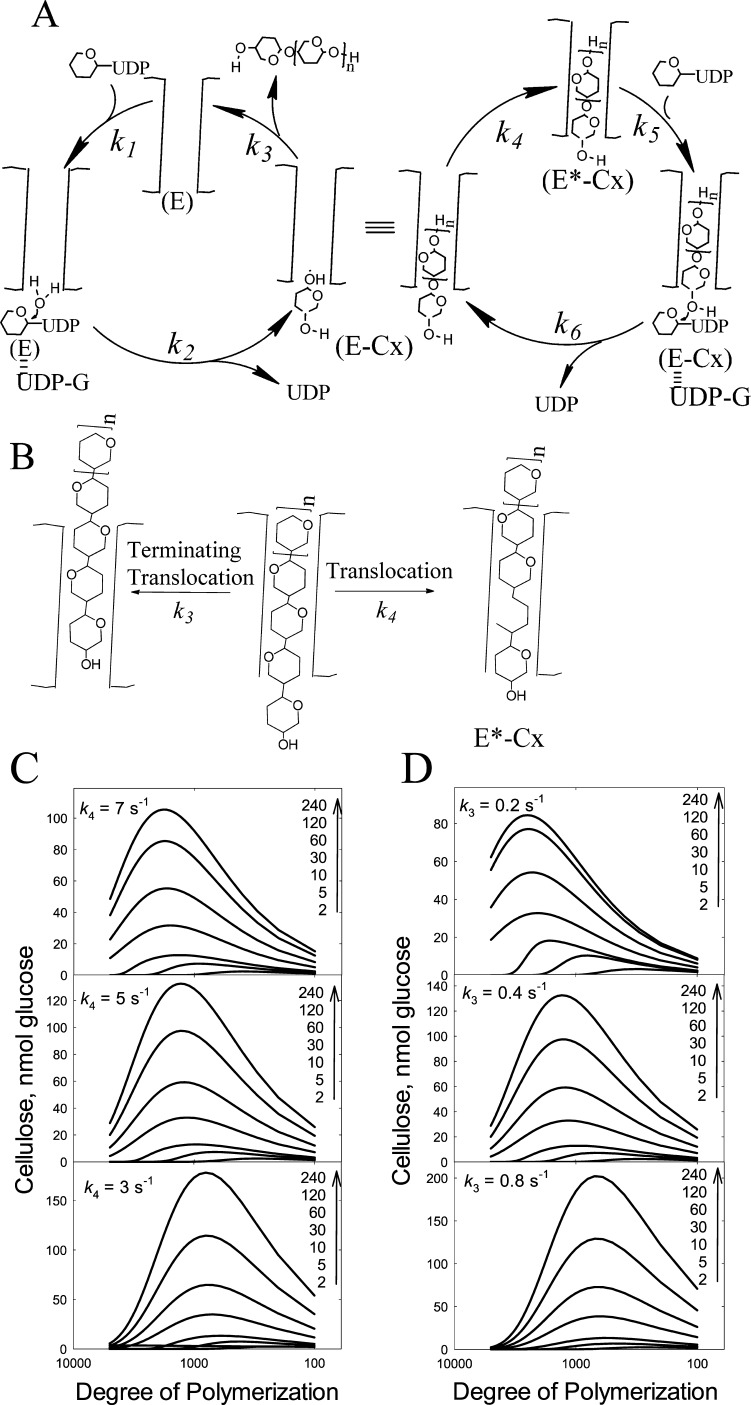
Figure 5.
Proposed mechanism for cellulose synthase and simulation of DOP. (A) Elongation starts with the cellulose chain properly positioned in the channel (E*-Cx) for nucleophile attack of UDP-Glc by the nonreducing end. UDP-Glc binds to E*-Cx (k5) to form the Michaelis complex. The attack of the 4-hydroxyl with the UDP-Glc forms the new glycosidic bond and E-Cx (k6). The newly added glucose at the UDP-Glc binding site then must translocate into the channel by one glucose unit forming E*-Cx (k4), which continues the cyclic process of elongation. Alternatively, translocation can proceed by more than one glucose unit resulting in strand release (k3) or termination forming free enzyme (E). The free enzyme (E) can still have a cellulose chain attached; however, it is not within close proximity to react with the incoming UDP-Glc. Initiation involves binding of UDP-Glc to E (k1). Hydrolysis of UDP-Glc yields glucose (k2). This intermediate is similar to the intermediate formed during elongation where the newly added glucose must translocate from the UDP-Glc binding site into the channel (k4). (B) A more detailed scheme. After addition of one glucose, the cellulose chain undergoes one translocation to form E*-Cx. Alternatively, the cellulose chain of E-Cx can undergo translocation greater than one glucose unit resulting in termination (k3). (C, D) Simulated elution profiles based on the mechanism shown in (A) and (B). Because Tenua can only accommodate 100 steps, the rate constants were multiplied by 100 for the purpose of illustrating the importance of the k4 to k3 ratio in determining the processivity of the enzyme. (C) shows that increasing k4 results in increased DOP. (D) shows that increasing k3 results in decreased DOP. The Tenua input in regard to the mechanism and the rate constants used for the simulations are provided in Figures S4 and S5 and Table 1.