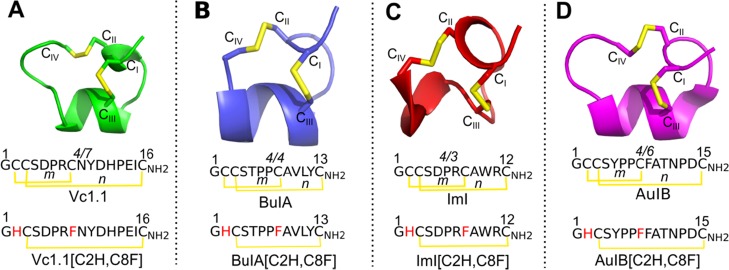
Figure 1.
NMR solution structures and sequences of α-conotoxins Vc1.1 (green), BuIA (blue), ImI (red), and AuIB (magenta) and the sequences of their disulfide-deleted analogues. (A) Vc1.1, (B) BuIA, (C) ImI, and (D) AuIB are small disulfide-rich (yellow) peptides belonging to the 4/7, 4/4, 4/3, and 4/6 α-conotoxin subfamilies, respectively. The CI–CIII disulfide bond of the α-conotoxins is deleted by replacing the Cys residues with His at position 2 and Phe at position 8. *m and n indicate the number of residues between CII–CIII and CIII–CIV disulfide bonds, respectively.