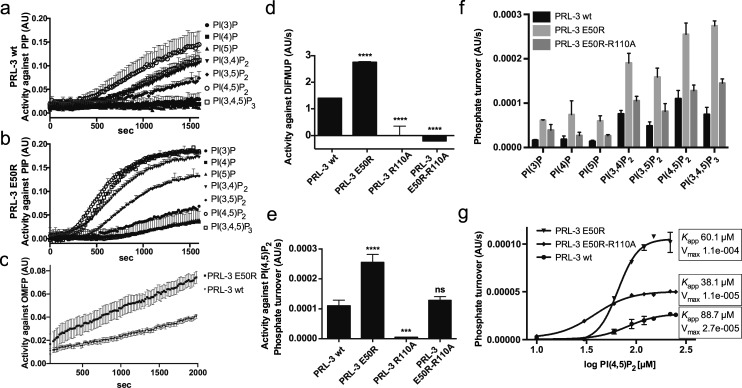
Figure 3.
Kinetic characterization of PRL-3 variant E50R. (a, b) PRL-3 variant (b) E50R dephosphorylates all PIP species more efficiently than (a) wild-type PRL-3, with the individual activity curves shown. (c) E50R shows increased activity against the unnatural substrate OMFP. (d–f) PRL-3 variant E50R-R110A reveals different roles of Arg50 for catalysis, depending on the substrate used. In vitro activities of PRL-3 variants against (d) DiFMUP and (e) PI(4,5)P2 are shown. Results are shown as mean ± SD. Statistics, compared to respective wt enzyme: ***p = 0.0004; ****p = 0.0001. (f) E50R-R110A dephosphorylates all seven PIP species with turnover values between E50R and wild-type PRL-3. (g) PI(4,5)P2 titration profiles reveal non-Michaelis–Menten kinetics with sigmoidal curve shapes, showing altering apparent affinity (Kapp) and maximal turnover for the different variants. Data are shown as mean ± SD. The following concentrations were used: PIPs 100 μM with 6 μM enzyme; OMFP 600 μM with 6 μM enzyme; and DiFMUP 21 μM with 50 nM enzyme.