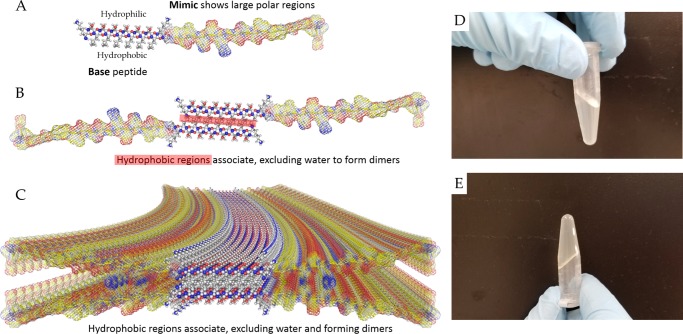
Scheme 1. Peptide Self-Assembly and Gross Hydrogel Structure.
(A–C) The base sequence has alternative hydrophilic and hydrophobic residues. In aqueous media, the hydrophobic leucine residues collapse into a core, exposing the hydrophilic serine residues to the surface. Stacking of a tetrameric unit to maximize hydrogen bonding leads to the formation of a β-sheet nanofiber. The lengthening and crosslinking of the nanofibers is favored in physiological salt concentrations as the repulsion among the positively charged lysine residues is shielded, leading to (D, E) the formation of robust hydrogels that maintain their shape.