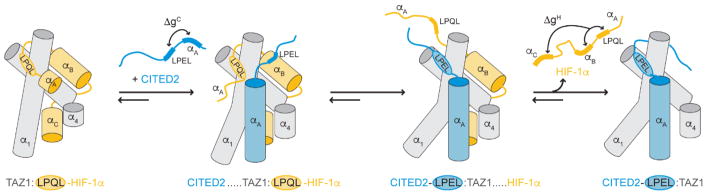
Figure 4.
Unidirectional allosteric regulation of the hypoxic response by CITED2. (Left) HIF-1α (orange) binds to the TAZ1 domain of CBP/p300 (gray) using three motifs (αA, LPQL-αB, and αC). (Middle left) The CITED2 αA helix (blue) binds to the TAZ1:HIF-1α complex, displacing the flexible HIF-1α αA motif. (Middle right) CITED2 further destabilizes the TAZ1:HIF-1α complex by competing for a shared binding site with its LPEL motif and triggering a conformational change in TAZ1. (Right) CITED2 successfully displaces HIF-1α to form a stable complex with TAZ1. All helices are represented as cylinders, with the α2 helix of TAZ1 omitted for clarity. ΔgC represents the thermodynamic coupling between the CITED2 αA and LPEL motifs. ΔgH represents the thermodynamic coupling between the LPQL-αB and αC motifs of HIF-1α. First published in Nature, vol. 543, p. 450, 2017 by Springer Nature.