Abstract
Cardiovascular devices and hemodynamic monitoring systems continue to evolve with the goal of allowing for rapid clinical intervention and management. Cardiovascular devices including the CardioMicroelectromechanical (CardioMEMS) device, implantable loop recorder, and right ventricular (RV) leadless pacemaker are now widely used for treatment and monitoring of advanced cardiac conditions, as many of these devices have been shown to significantly improve patient outcomes. Additionally, hemodynamic monitoring devices have shown utility in monitoring patients with aortic aneurysms after endovascular aortic repair (EVAR) for early detection of Type I and Type II endoleaks. There is limited published data regarding the imaging features of these devices. As these devices become more widely used, it is important for radiologists to become familiar with the normal imaging features and potential complications. The goal of this review is to summarize the data regarding the use of leadless cardiovascular devices including the CardioMEMS device, implantable loop recorder, and RV leadless pacemaker, and to present cases demonstrating their utility and normal imaging features.
Rapid growth in microcircuit and battery technology has led to the development of several leadless cardiovascular devices, which are now increasingly being used in patient care (1). Leadless cardiac devices including the CardioMicroelectromechanical (CardioMEMS) device, implantable loop recorder, and right ventricular (RV) leadless pacemaker are important tools in the treatment and management of patients with common cardiac conditions including heart failure, cardiac syncope, and arrhythmias. New cardiac hemodynamic and electrocardiography monitoring devices are capable of providing information for early intervention and clinical management (2). Additionally, wireless devices such as wireless pacemakers offer a minimally invasive option for patients with decreased risk, which may improve patient satisfaction and outcomes (3). In addition to patients with cardiac conditions, devices such as the CardioMEMS device have shown utility in the monitoring of patients with aortic aneurysms after endovascular aortic repair (EVAR) for early detection of endoleaks. These leadless cardiac, venous, aortic, and subcutaneous devices depend on correct anatomic position for proper function, which is evaluated with radiologic imaging. As these devices become more commonly used in clinical practice, the radiologists’ understanding of these devices and their utility is paramount for patient follow-up and detection of potential complications. The goal of this review is to summarize the data regarding the use of CardioMEMS device, implantable loop recorder, and RV leadless pacemaker, and to demonstrate their normal imaging features.
CardioMEMS
In the United States, heart failure accounts for over 1 million hospitalizations per year and an overall annual cost of $30.7 billion (4). Advances in heart failure clinical management have improved survival with heart failure; however, morbidity and mortality remain high. The CardioMEMS device (St. Jude Medical, Inc.) was developed as a permanently implanted wireless device for continuous hemodynamic monitoring in patients with heart failure. Previous studies have shown that clinical signs and symptoms of heart failure as well as laboratory values including N-terminal pro-brain natriuretic peptide (NT-BNP) and pro-brain natriuretic peptide (BNP) fail to recognize early signs of heart failure (5). The effectiveness of the CardioMEMS device in guiding clinical management was evaluated with the landmark CHAMPION study in 2011 which demonstrated that pulmonary artery pressure-based therapy reduces hospitalizations and mortality (6). The CHAMPION study was a multicenter randomized, prospective, single-blinded trial that included 550 patients at 64 sites in the United States. In contrast to prior heart failure hemodynamic monitoring studies, the CHAMPION trial provided specific recommendations for heart failure management based on hemodynamic monitoring using American College of Cardiology and American Heart Association guidelines. The primary outcomes of the CHAMPION study showed that hemodynamic-based therapy resulted in a relative risk reduction in the number of hospitalizations by 28% at 6 months and 37% at 15 months. The study showed a decrease in mortality by 57% and improved quality of life among heart failure patients. Subsequent studies also showed that in advanced heart failure cases, pulmonary artery pressure-based therapy decreased time to left ventricular assist device (LVAD) placement and cardiac transplant (7).
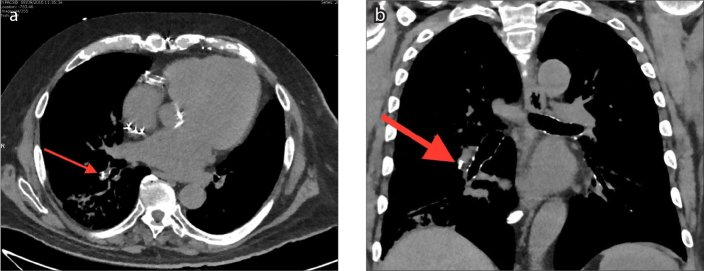
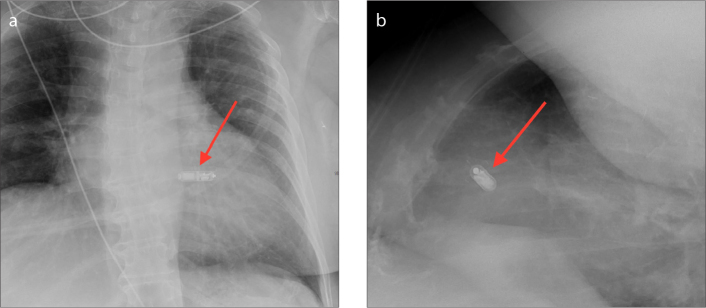
The CardioMEMS device is an externally powered, leadless wireless sensor. The wireless sensor measures 2×3.4×15 mm and has two nitinol sensor wire loops attached, which maintain position within the vessel (Fig. 1). The total length of the device including the wires measures 4.5 cm. The device is delivered via a minimally invasive catheter-based delivery system under fluoroscopy. Anteroposterior (AP) and lateral chest radiographs are routinely performed postoperatively to confirm sensor location. The radiopaque wireless sensor is visible on conventional radiographs (Fig. 2); however, the wire loops are not typically visualized (8). Both the sensor and loops are visible on computed tomography (CT) (Fig. 3). The sensor is usually inserted in the lower lobe region of the left pulmonary artery (Figs. 2, 3); however, it can also be inserted into branches of the right pulmonary artery (Figs. 4, 5). The sensor appears on radiographs and CT as a linear metallic device within the pulmonary artery. The CardioMEMS device is considered MRI-conditional. Adverse effects of the CardioMEMS device are rare with an overall complication-free rate of 98.6%; however, complications are typically related to placement of the wireless sensor (5). The most common complication during placement of the CardioMEMS device is retrograde movement of the device after deployment (9). This can be visualized on postoperative radiographs or CT imaging. Complications include incomplete deployment of the sensor, hemorrhage, infection, and thrombus. Typical follow-up and evaluation for potential complications includes serial CT imaging. Specific attention should be made on both radiographs and CT comparing the position of the sensor with immediate postoperative position to identify potential sensor displacement.
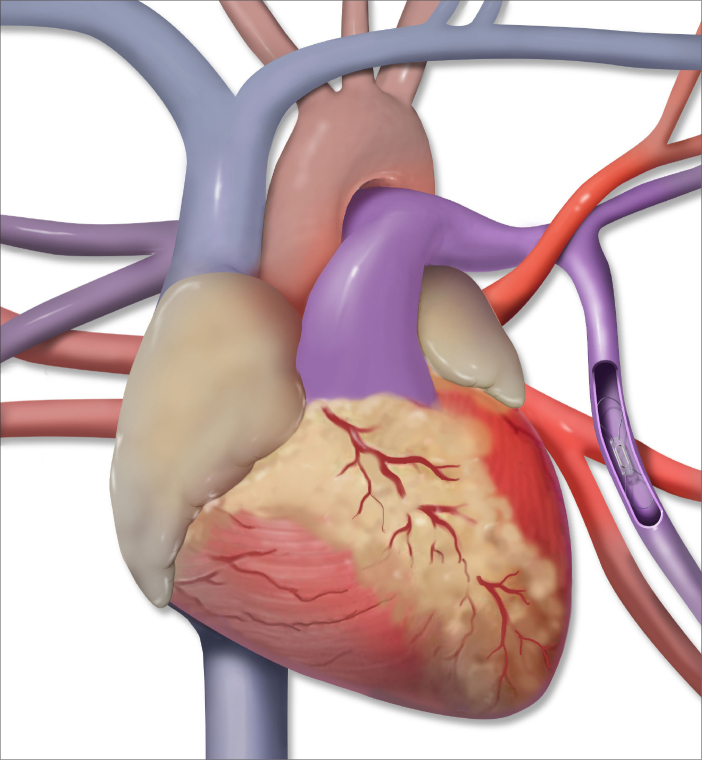
Figure 1.
Illustration of the CardioMEMS device positioned in the left lower lobe segmental pulmonary artery.
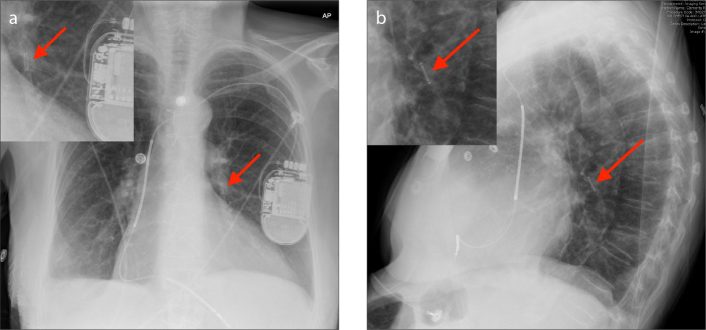
Figure 2 a, b.
AP with zoomed image (a) and lateral with zoomed image (b) radiographs of the CardioMEMS device inserted in the left lower lobe segmental pulmonary artery (arrows).
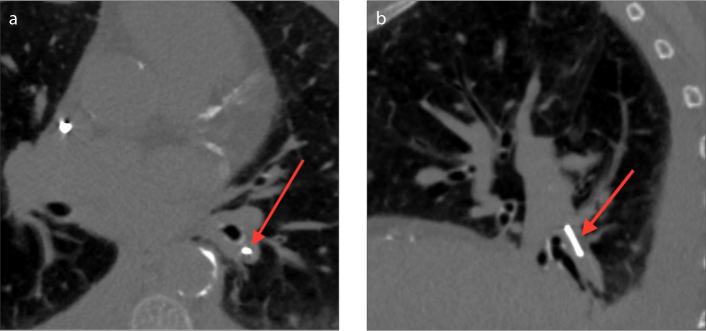
Figure 3 a, b.
Axial (a) and sagittal (b) CT images of the CardioMEMS device inserted in the left lower lobe segmental pulmonary artery (arrows).
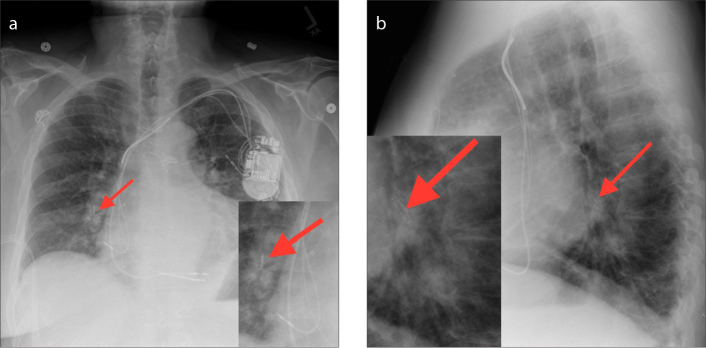
Figure 4 a, b.
AP with zoomed image (a) and lateral with zoomed image (b) chest radiographs showing the CardioMEMS device inserted in the right lower lobe lobar pulmonary artery (arrows).
Figure 5 a, b.
Axial (a) and coronal (b) CT images showing the CardioMEMS device inserted in the right lower lobe segmental pulmonary artery (arrows).
CardioMEMS in aortic aneurysms
While the CardioMEMS device is FDA approved for use in heart failure, studies have also shown the utility of hemodynamic monitoring devices in patients with aortic aneurysms after endovascular aortic repair (EVAR). The device is currently considered investigational in patients with prior EVAR, but preliminary studies have demonstrated utility in early detection of Type I and Type II endoleaks. In a study by Mehta, et al. (10), 480 patients underwent endovascular aortic repair with simultaneous placement of CardioMEMS device for postsurgical hemodynamic monitoring. This study showed significantly increased systolic pressure, diastolic pressure, and pulse pressure indexes of the excluded aortic sac in patients with Type I and Type II endoleaks compared with patients with no endoleaks. In addition, all of these pressure indexes were significantly reduced following successful treatment of the endoleaks. This data suggests that pressure sensor devices such as CardioMEMS device may be a useful additional tool for early diagnosis and management of endoleaks (11).
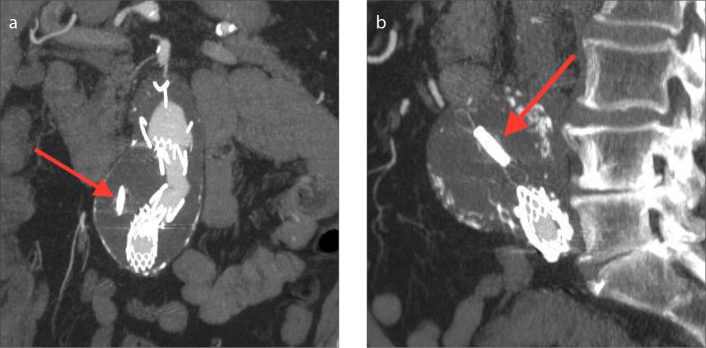
The imaging features of the CardioMEMS device in patients with aortic aneurysms after EVAR are similar to those in patients with heart failure; however, the sensor is inserted within the aneurysmal sac adjacent to the stent graft (Figs. 6–8). As with the CardioMEMS device in heart failure, complications are typically related to device placement or device migration.
Figure 6.
Illustration of the CardioMEMS device inserted in an excluded aortic aneurysm status post endovascular aortic repair.
Figure 7.
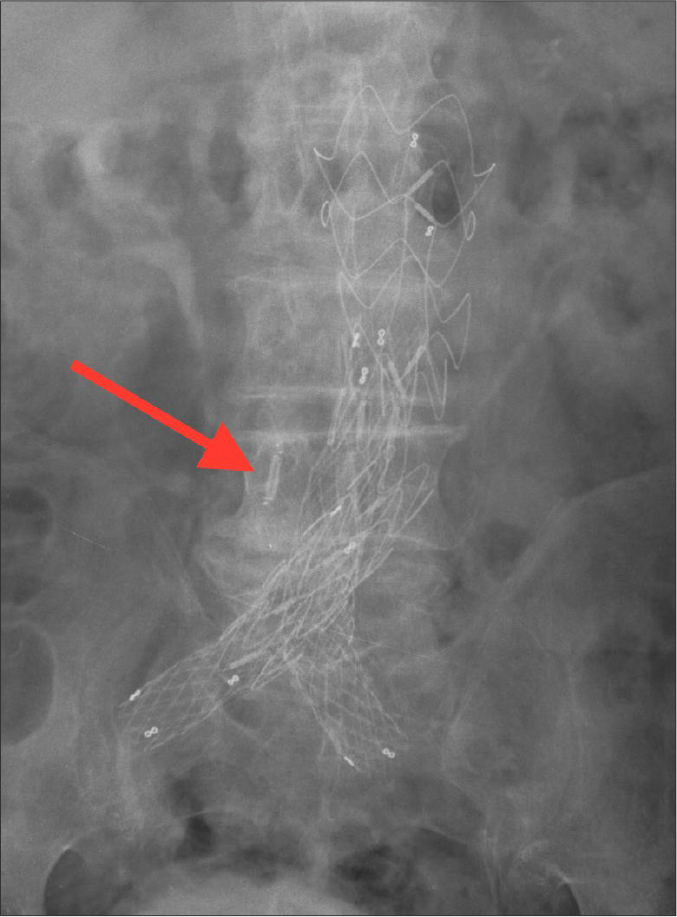
AP abdominal radiograph of the CardioMEMS device inserted in an excluded aortic aneurysm status post endovascular aortic repair.
Figure 8 a, b.
Coronal (a) and sagittal (b) MIP CT images of CardioMEMS device inserted in an excluded aortic aneurysm status post endovascular aortic repair (arrows).
Implantable loop recorder
In addition to advances in heart failure and aortic aneurysm monitoring, technologic advances have been made in the workup and management of patients with syncope using implantable loop recorders (ILRs). Syncope accounts for approximately 3.5% of emergency room visits, and 1%–6% of hospital admissions (12). Cardiac arrhythmia is an important cause of syncope; however, it is often difficult to diagnose, and documentation of an arrhythmia responsible for symptoms can be challenging (13). The current gold standard of diagnosing cardiac syncope is correlation with electrocardiography (ECG). Implantable loop recorders allow for prolonged ECG monitoring and can be used in monitoring patients with unexplained syncope or assessing patients with known arrhythmias to help guide clinical management (12). Many observational trials and four retrospective trials have shown ILRs to be useful in the early diagnosis of unexplained syncope (14). In the Randomized Assessment of Syncope Trial (RAST), for example, the use of ILRs resulted in a diagnosis in 52% of patients compared with 20% of patients using conventional workup techniques (15). Although ILRs have been shown to be effective in diagnosis of cardiac syncope, no studies have demonstrated a mortality benefit.
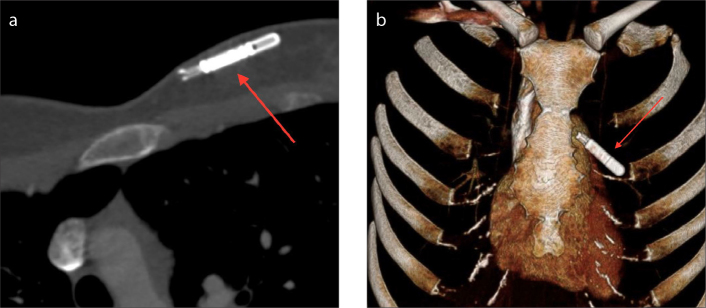
The ILRs that are currently in clinical use include the Reveal DX, XT, Plus, and LINQ by Medtronic and the Confirm by St. Jude Medical. These devices measure 62×19×8 mm and are usually implanted subcutaneously over the left pectoral muscle under local anesthesia. They contain a pair of sensing leads that record ECG data, which is downloaded via radiofrequency (12). All of these devices are considered MRI-conditional. On radiographs and CT, the implantable loop recorder appears as a rectangular density localized to the pre-sternal subcutaneous tissues (Fig. 9). Since they are superficially inserted, ILRs have a very low risk of complication. Major complications include postinsertion hematoma and infection.
Figure 9 a, b.
Axial CT (a) and 3D volume rendering (b) images of an implantable loop recorder (Medtronic LINQ) inserted in the left chest wall (arrows).
Right ventricular leadless pacemakers
In addition to hemodynamic monitoring devices, significant advances have been made in the development of RV wireless pacemakers. Pacemakers have long since been established as the treatment of choice in patients with symptomatic bradyarrythmias, as they have been shown to reduce symptoms and decrease morbidity and mortality (16). Traditional pacemakers consist of a subcutaneous pulse generator inserted into the chest wall and transvenous pacing leads attached to the myocardium. However, this device system is associated with specific complications related to the subcutaneous generator and transvenous leads. The most significant complications include pneumothorax, hematoma, lead fracture due to mechanical stress, venous thrombosis, and infection (17). Overall, the rate of complication from cardiac pacing is estimated to be about 10% (17). Leadless pacemaker systems were developed to minimize risks, improve patient outcomes, and offer a minimally invasive option for patients requiring single chamber pacemaker placement. The safety and efficacy of leadless cardiac device placement was demonstrated in the LEADLESS II trial, which demonstrated an overall complication-free rate of 94% (18). The LEADLESS II trial was a prospective, non-randomized, single-arm, multicenter trial including 526 patients requiring single-chamber transcutaneous ventricular pacing (19). Of the 526 patients in the trial, 504 had successful placement of a wireless cardiac device resulting in a rate of successful implantation of 95.8%. At six months, 90% of patients met the primary efficacy goal for pacing and sensing, which is similar to conventional pacemakers.
There are currently two right ventricular leadless pacemaker systems available for clinical use: Nanostim™ Leadless Pacemaker System (LPS, St. Jude Medical) and the Micra™ Transcatheter Pacing System (TPS, Medtronic). Both systems are placed via femoral venous access and implanted directly into the right ventricular wall (16). While the two models vary slightly in dimensions, both are about the size and shape of a AAA battery (20). The Nanostim™ device has a small screw-in helix for fixation to the right ventricular tissues, whereas the Micra™ device contains small nitinol tines for fixation. Both models are MRI-conditional. On radiographs and CT, RV leadless pacemakers appear as a linear radiopaque device implanted into the right ventricular wall (Figs. 10, 11). Potential complications include device dislodgement, cardiac perforation, elevated pacing thresholds requiring device retrieval and reimplanatation, and vascular complications. Dislodgement and cardiac perforation can be identified on radiographs, and vascular complications can be assessed on CT imaging.
Figure 10 a, b.
AP (a) and lateral (b) chest radiographs showing an RV leadless pacemaker (Medtronic Micra) inserted in the right ventricle.
Figure 11 a, b.
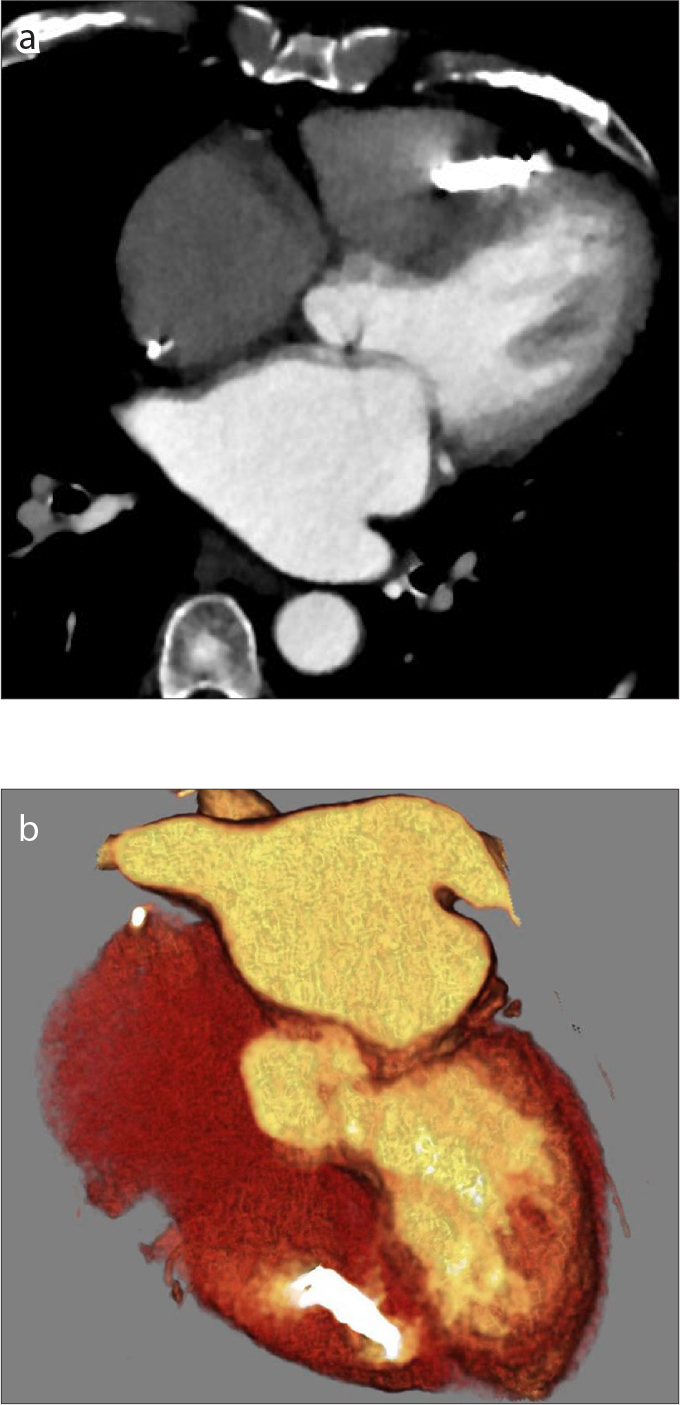
Axial CT image (a) and oblique coronal 3D volume rendering image (b) showing an RV wireless pacemaker (Medtronic Micra) inserted in the right ventricle.
In addition to right ventricular epicardial pacing, the Wireless Cardiac Stimulation in Left Ventricle (WiCS-LV) system has been developed for left ventricular endocardial pacing in patients with failed cardiac resynchronization (21). Wireless endocardial left ventricular (LV) pacing has advantages including more physiologic pacing, enhanced systolic and diastolic function, lower potential for arrhythmia, and lower pacing output requirements (22). This system consists of a subcutaneous pulse generator and a receiver electrode implanted into the left ventricular endocardium. However, using this system patients still require concomitant conventional implantable right ventricular pacing devices, as the left ventricular endocardial pacing system is triggered from a conventional right ventricular pacing spike (21, 22). In the Wireless Stimulation Endocardially for CRT (WiSE-CRT) study, preliminary results showed the system improved left ventricular function at 6 months; however, the study was terminated prematurely due to safety concerns including inability to successfully implant the device, pericardial effusions due to device delivery technique, loss of biventricular pacing, and depletion of the battery (22). Additional studies have been performed with device system modifications; however, these studies had additional safety concerns including increased thromboembolic events (22). Larger studies are required before the WiCS-LV system becomes commonly used in clinical practice.
Conclusion
Leadless cardiac devices continue to evolve. As the use of these devices becomes more widespread, it is important for radiologists to become familiar with the normal imaging features of these devices and their potential complications.
Main points.
Leadless cardiac devices including the CardioMEMS device, implantable loop recorder, and RV leadless pacemaker are important tools in the treatment and management of patients with common cardiac conditions such as heart failure, cardiac syncope, and arrhythmias.
These leadless cardiac and venous devices depend on correct anatomic position for proper function, which is evaluated with radiologic imaging.
As these devices become more commonly used in clinical practice, the radiologists’ understanding of these devices and their utility is paramount for patient follow-up and detection of potential complications.
Footnotes
Conflict of interest disclosure
The authors declared no conflicts of interest.
References
- 1.Sperzel J, Burri H, Gras D, et al. State of the art of leadless pacing. Europace. 2015;17:1508–1513. doi: 10.1093/europace/euv096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yandrapalli S, Raza A, Tariq S, Aronow WS. Ambulatory pulmonary artery pressure monitoring in advanced heart failure patients. World J Cardiol. 2017;9:21–26. doi: 10.4330/wjc.v9.i1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ricci RP, Morichelli L, Quarta L, et al. Long-term patient acceptance of and satisfaction with implanted device remote monitoring. EP Europace. 2010;12:674–679. doi: 10.1093/europace/euq046. [DOI] [PubMed] [Google Scholar]
- 4.Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart Disease and Stroke Statistics—2017 Update: A Report From the American Heart Association. Circulation. 2017;136:e1–e23. doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tang WH, Girod JP, Lee MJ, et al. Plasma B-type natriuretic peptide levels in ambulatory patients with established chronic symptomatic systolic heart failure. Circulation. 2003;108:2964–2966. doi: 10.1161/01.CIR.0000106903.98196.B6. [DOI] [PubMed] [Google Scholar]
- 6.Abraham WT, Adamson PB, Bourge RC, et al. Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: a randomised controlled trial. Lancet. 2011;377:658–666. doi: 10.1016/S0140-6736(11)60101-3. [DOI] [PubMed] [Google Scholar]
- 7.Feldman D, Naka Y, Cabuay B, et al. A wireless hemodynamic pressure sensor before and after ventricular assist device placement: A sub-study of the CHAMPION trial. J Heart Lung Transplant. 2011;30:S86. doi: 10.1016/j.healun.2011.01.248. [DOI] [Google Scholar]
- 8.Lichtenberger JP, Hui Gladwin, Carter BW, Jamis-Dow C, Abbara S. Cardiac Devices. In: Abbara S, Kalva S, editors. Problem solving in cardiovascular imaging. 1st ed. Philadelphia: Elsevier; 2013. pp. 298–312. [Google Scholar]
- 9.Shavelle D, Jermyn R. The CardioMEMS heart failure sensor: a procedural guide for implanting physicians. J Invasive Cardiol. 2016;17:273–279. [PubMed] [Google Scholar]
- 10.Mehta M, Taggert JB, Roddy SP, et al. Implications of endoleaks on aneurysm sac pressures following endovascular repair of elective and ruptured aortic aneurysms. J Vasc Surg. 2009;49:S7. doi: 10.1016/j.jvs.2009.02.150. [DOI] [Google Scholar]
- 11.Gandhi RT, Katzen BT, Tsoukas AI, Geisbüsch P. Aortic Aneurysm pressure sensors can be of value in the acute postoperative setting. Vasc Endovascular Surg. 2011;45:412–417. doi: 10.1177/1538574411408741. [DOI] [PubMed] [Google Scholar]
- 12.Kanjwal K, Figueredo VM, Karabin B, Grubb BP. The implantable loop recorder: current uses, future directions. J Innov Card Rhythm Manage. 2011;2:215–222. [Google Scholar]
- 13.Ermis C, Zhu AX, Pham S, et al. Comparison of automatic and patient-activated arrhythmia recordings by implantable loop recorders in the evaluation of syncope. Am J Cardiol. 2003;9:815–819. doi: 10.1016/S0002-9149(03)00889-0. [DOI] [PubMed] [Google Scholar]
- 14.Tanno K. Use of implantable and external loop recorders in syncope with unknown causes. J Arrhythm. 2017;33:579–582. doi: 10.1016/j.joa.2017.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krahn AD, Klein GJ, Yee R, Skanes AC. Randomized assessment of syncope trial: conventional diagnostic testing versus a prolonged monitoring strategy. Circulation. 2001;104:46–51. doi: 10.1161/01.CIR.104.1.46. [DOI] [PubMed] [Google Scholar]
- 16.Brunner M, Olschewski M, Geibel A, Bode C, Zehender M. Long-term survival after pacemaker implantation: Prognostic importance of gender and baseline patient characteristics. Eur Heart J. 2004;25:88–95. doi: 10.1016/j.ehj.2003.10.022. [DOI] [PubMed] [Google Scholar]
- 17.Sideris S, Archontakis S, Dilaveris P, et al. Leadless cardiac pacemakers: current status of a modern approach in pacing. Hellenic J Cardiol. 2017;58:403–410. doi: 10.1016/j.hjc.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 18.Reddy VY, Knops RE, Sprezel J, et al. permanent leadless cardiac pacing: results of the LEADLESS trial. Circulation. 2017;136:1466–1471. doi: 10.1161/CIRCULATIONAHA.113.006987. [DOI] [PubMed] [Google Scholar]
- 19.Reddy VY, Exner DV, Cantillon DJ, et al. Percutaneous implantation of an entirely intracardiac leadless pacemaker. N Engl J Med. 2015;373:1125–1135. doi: 10.1056/NEJMoa1507192. [DOI] [PubMed] [Google Scholar]
- 20.Torres-Ayala SC, Santacana-Laffitte G, Maldonado J. Radiography of cardiac conduction devices: a pictoral review of pacemakers and implantable cardioverter defibrillators. J Clin Imaging Sci. 2014;4:74. doi: 10.4103/2156-7514.148269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seifert M, Butter C. Evaluation of wireless stimulation of the endocardium, WiSE, technology for treatment heart failure. Expert Rev Med Devices. 2016;13:523–531. doi: 10.1080/17434440.2016.1187559. [DOI] [PubMed] [Google Scholar]
- 22.Miller MA, Neuzil P, Dukkipati SR, Reddy VY. leadless cardiac pacemakers: back to the future. J Am Coll Cardiol. 2015;66:1179–1189. doi: 10.1016/j.jacc.2015.06.1081. [DOI] [PubMed] [Google Scholar]