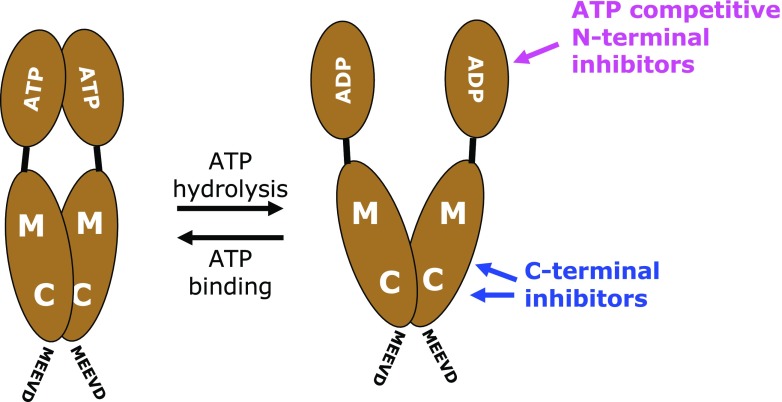
Fig. 2.
General scheme of the domain structure of Hsp90 and of the target domains of the main classes of Hsp90 inhibitors. ADP and ATP illustrate the importance of the N-terminal domain for ATP binding and hydrolysis; M and C, middle and C-terminal domains; MEEVD, very C-terminal pentapeptide (hallmark of cytosolic Hsp90 isoforms) and binding site for most of the co-chaperones containing tetratricopeptide repeats