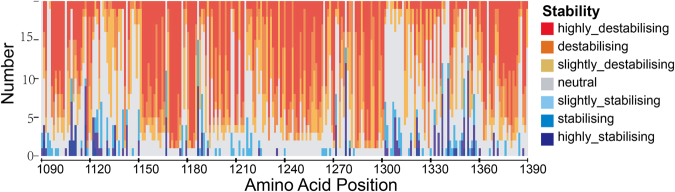
Figure 1.
Effect of mutations on ALK kinase domain stability. All 19 possible mutations along with the synonymous mutations that the residue mutates to itself at each position in ALK kinase domain are colored on a vertical bar in terms of their stability relative to wide-type ALK. The values of ΔΔGfold are binned into seven categories: highly stabilizing (ΔΔGfold < −1.84 kcal·mol−1) and highly destabilizing (ΔΔGfold > 1.84 kcal·mol−1); stabilizing (−1.84 kcal·mol−1 < ΔΔGfold < −0.92 kcal·mol−1) and destabilizing (0.92 kcal·mol−1 < ΔΔGfold < 1.84 kcal·mol−1); slightly stabilizing (−0.92 kcal·mol−1 < ΔΔGfoldd < −0.46 kcal·mol−1) and slightly destabilizing (0.92 kcal·mol−1 < ΔΔGfold < 1.84 kcal·mol−1); and neutral (−0.46 kcal kcal·mol−1 < ΔΔGfold < 0.46 kcal·mol−1).