Fig. 3|. Typhoid toxin binds preferentially to Neu5Ac displayed in the context of multiantennal N-glycans over linear N-glycans.
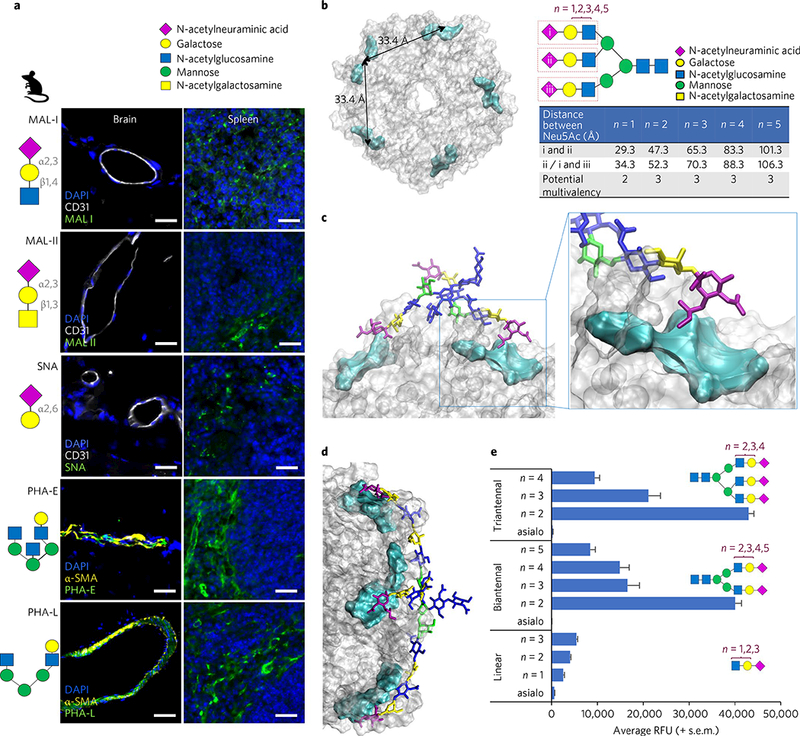
Glycan symbol and colour coding recommended by the Consortium for Functional Glycomics are used. a, Glycan binding preferences of five plant lectins, indicated schematically. Unlike the others, PHA-E and -L lectins phenocopied the in vivo tropism of typhoid toxin, indicating that endothelial cells of arterioles in the brain and immune cells express multiantennal N-glycans. Shown are representative images of at least two independent experiments. Mouse numbers used are: Mal-I (n = 2), Mal-II (n = 2), SNA (n = 2), PHA-E (n = 4) and PHA-L (n = 4). Scale bars, 100 μm. b, Top view of the PltB homopentamer. Cyan: location of the receptor binding pocket in the PltB subunits. The two adjacent binding pockets are separated by 33.4 Å. The distance is measured between Neu5Acs of the triantennal glycan with LacNAc sialosides (n = 1,2,3,4,5). In the schematic depicted in b, triantennal N-glycan contains the trisaccharide consensus recognized by typhoid toxin (red rectangles)9. n = 1,2,3,4,5, elongated forms of N-acetyllactosamine (LacNAc; galactose β1–4 linked to N-acetylglucosamine). c,d, Depiction of the mono-LacNAc sialoside interacting with two receptor binding sites within a single homopentamer (c), while the bi-LacNAc sialoside interacts with three receptor binding sites (d). e, Glycan microarray indicating that triantennal, bi-LacNAc sialoside (no. 61, the 61st glucan in the microarray that contains 130 glycans) shows the strongest binding to typhoid toxin (see Supplementary Fig. 7 for the results for all 130 glycans). These results also imply that sialosides with the unnecessarily long extension of LacNAc are unlikely to be favourable for typhoid toxin binding. Glycans shown are linear glycans (asialo (glycan with no terminal Neu5Ac) (no. 1), n = 1 (no. 17), n = 2 (no. 18), n = 3 (no. 19)), biantennal glycans (asialo (no. 7), n = 2 (no. 55), n = 3 (no. 56), n = 4 (no. 57), n = 5 (no. 58)) and triantennal glycans (asialo (no. 9), n = 2 (no. 61), n = 3 (no. 62), n = 4 (no. 63)). Data presented as mean±s.e.m.
