Figure 5.
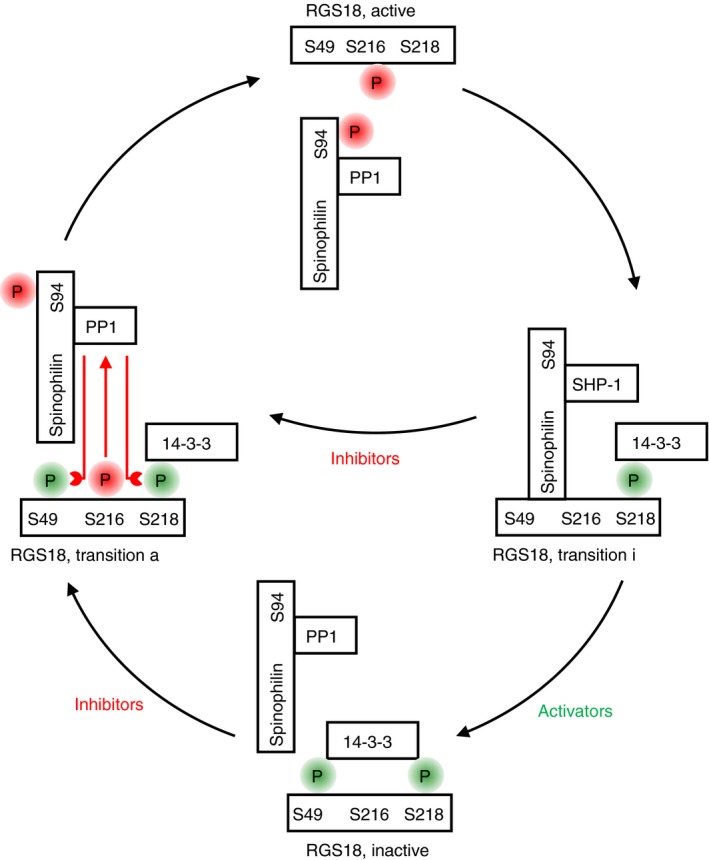
Activation/inactivation cycle of RGS18. Regulator of G‐protein signaling 18 (RGS18) terminates Gq signaling and is regulated by both platelet activating and inhibitory pathways. In freshly isolated resting platelets RGS18 is found in a complex with the adapter protein spinophilin, the tyrosine phosphatase SHP‐1 and the phospho‐serine/threonine binding protein 14‐3‐3γ attached to phosphorylated serine 218 of RGS18 (transition state on the way towards inactivation, transition i). Platelet activators like thrombin and TXA 2 induce the phosphorylation of serine 49 of RGS18 generating a second 14‐3‐3 binding site leading to enhanced 14‐3‐3 binding. Simultaneously, SHP‐1 is activated and detaches from spinophilin (involving de‐phosphorylation of tyrosine residues on spinophilin), the serine/threonine phosphatase PP1 binds to spinophilin instead, and spinophilin dissociates from RGS18. In this state the RGS18 complex is inactive. Platelet inhibitors like prostacylin and nitric oxide induce the phosphorylation of serine 216 on RGS18 and serine 94 on spinophilin which lead to the activation of PP1 and detachment of spinophilin from RGS18 (transition state on the way towards activation, transition a). Active PP1 removes phosphate groups from serines 49 and 218 of RGS18 leading to the loss of 14‐3‐3 binding. This state of the RGS18 complex is characterized by free catalytically active RGS18 which can hydrolyse Gαq‐GTP to form inactive Gαq‐GDP (Figure 4). Phosphorylation sites linked to platelet inhibition are highlighted in red, whereas sites linked to platelet activation are marked in green
