Figure 7.
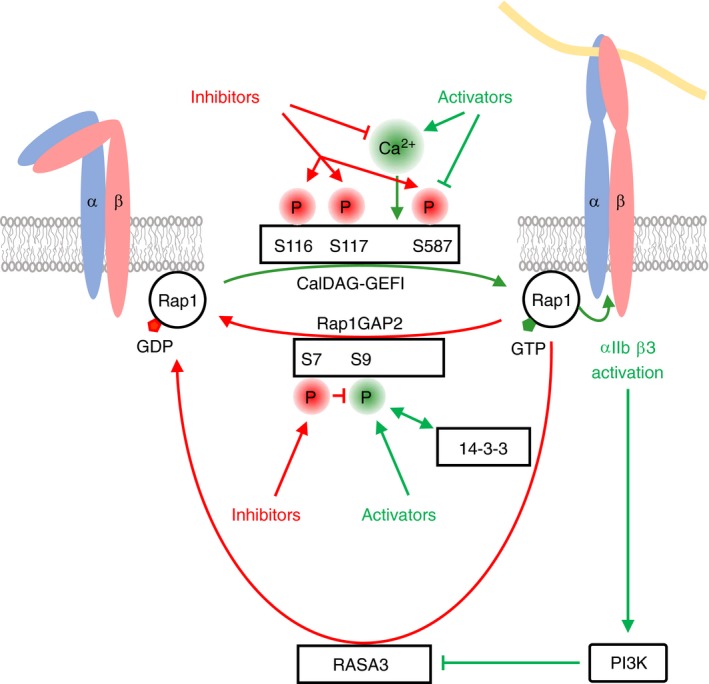
Regulation of Rap1 by GEFs and GAPs. Integrin αIIbß3 is a major platelet integrin required for platelet aggregation. The small G‐protein Rap1 is a positive regulator of integrin αIIbß3 activation and cycles between an inactive, GDP‐bound and an active, GTP‐bound state. Formation of Rap1‐GTP and integrin activation are enabled by the calcium ion (Ca2+) dependent guanine‐nucleotide exchange factor CalDAG‐GEFI, whereas hydrolysis of Rap1‐GTP to inactive Rap1‐GDP requires the GTPase‐activating proteins (GAPs) Rap1GAP2 and RASA3. Rap1GAP2 interacts with the phospho‐serine/threonine binding protein 14‐3‐3 through phosphorylated serine 9 on Rap1GAP2. During platelet activation levels of free intracellular Ca2+ rise (Figure 4) leading to the activation of CalDAG‐GEFI, increased Rap1‐GTP formation and integrin activation. In contrast, cyclic nucleotide‐dependent inhibitory pathways on one hand suppress intracellular Ca2+ levels, thus indirectly inhibit CalDAG‐GEFI activation, and on the other hand result in phosphorylation of CalDAG‐GEFI on serines 116, 117 and 587 inhibiting its activity. Simultaneously, cyclic nucleotide‐dependent inhibitory pathways induce Rap1GAP2 phosphorylation on serine 7 leading to an inhibition of 14‐3‐3 binding, activation of Rap1GAP2, reduced Rap1‐GTP levels and lowered Rap1 activity. In contrast, platelet activation leads to phosphorylation of Rap1GAP2 on serine 9 resulting in enhanced 14‐3‐3 binding and inhibition of Rap1GAP2. In parallel, αIIbβ3‐mediated outside‐in signaling leads to phosphatidylinositol 3‐kinase (PI3K) activation, which in turn reduces the GAP activity of RASA3. Platelet activators also suppress the phosphorylation of the inhibitory serine 587 site on CalDAG‐GEFI. The consequence of Rap1GAP2 and RASA3 inhibition, along with CalDAG‐GEFI stimulation is enhanced Rap1 activity. Phosphorylation sites linked to platelet inhibition are highlighted in red, whereas sites linked to platelet activation are marked in green
