Abstract
Background
Anticoagulation control with vitamin‐K antagonists (VKAs) in patients with atrial fibrillation (AF) or venous thromboembolism (VTE) can be measured using time in therapeutic range (TTR), where TTR >65% is considered good and low TTR may be associated with low adherence.
Methods
This cross‐sectional observational study compared illness beliefs, treatment beliefs, and treatment satisfaction of patients with TTR >75% and TTR <50% using validated tools to determine their association with TTR. Adults requiring chronic VKA therapy were recruited from 2 hospital anticoagulation clinics in London, UK.
Results
311 patients with TTR >75% and 214 with TTR <50% were recruited. TTR >75% patients had been taking warfarin on average over 2 years longer than TTR <50% patients (P < .001). Statistically significant differences in beliefs were found in all subscales other than in treatment control, general harm, and general overuse. Cluster analysis determined there were 4 distinct clusters of beliefs among patients. Multivariate binary logistic regression found VTE patients were least likely to have poor TTR (OR = 0.49; 95% CI 0.29, 0.77). Patients in the “cautious of therapy and fearful of illness” cluster were most likely to have low TTR (OR = 4.75; 95% CI 2.75, 8.77).
Conclusion
Illness perceptions, medication beliefs and treatment satisfaction were associated with INR control. VTE patients and those who were accepting of both illness and treatment were most likely to have optimal INR control.
Keywords: adherence, anticoagulation, atrial fibrillation, illness belief, medication beliefs, quality of life, time in therapeutic range, venous thromboembolism, vitamin‐K antagonists, warfarin
Essentials.
Study comparing illness beliefs, treatment beliefs and treatment satisfaction according to TTR.
TTR <50% associated with negative health beliefs compared to TTR >75%.
Cluster analysis generated belief profiles, where different profiles were associated with TTR.
Indicates beliefs that can be targets for interventions to improve adherence and TTR.
1. INTRODUCTION
Guidelines recommend lifelong anticoagulation in atrial fibrillation (AF) in the presence of stroke risk factors,1, 2 as well as in the secondary prevention of venous thromboembolism (VTE).3, 4 Nonadherence to anticoagulation therapy is known to impact treatment outcomes,5, 6 where adherence is defined as the extent to which a patient takes their medication as prescribed.7
Time in therapeutic range (TTR) using the Rosendaal method indicates international normalized ratio (INR) control, where a TTR >65% is considered good and indicative of treatment adherence to vitamin‐K antagonists (VKAs).2, 8, 9 Low TTR has been shown to be associated with both poor adherence and worse clinical outcomes.10, 11 Long‐term VKA therapy presents unique challenges regarding medication adherence where patients may consider them cumbersome due to regular laboratory monitoring of INR, frequent dose changes, and variable dosing regimens while being restrictive regarding other drugs and foods. These factors may affect TTR,12, 13 as well as treatment satisfaction and quality of life (QoL).14 As TTR is routinely available to clinicians, it provides a means by which adherence can be objectively assessed.
Nonadherence is prevalent in chronic disease where up to 50% of patients are reportedly nonadherent.15, 16 Unintentional nonadherence results from a lack of internal resources (capacity) or external resources (practical factors) inhibiting adherence. Intentional nonadherence involves a conscious underlying perceptual or motivational barrier to adherence, such as patient beliefs about an illness or medicines.17, 18 Furthermore, behavior is influenced by one’s capability to adhere, opportunity to adhere, and motivation to adhere, as described in the COM‐B model of adherence, each factor being potentially modifiable.19, 20, 21 Among those requiring chronic anticoagulation, patients may be asymptomatic and medication use is purely preventative. Therefore, drivers to adhere can be lacking.22
While extensive research has been conducted to determine adherence to VKAs,22 there is a paucity of research determining the impact of beliefs and QoL on adherence to VKAs and TTR. The Switching Study is a program of work investigating the association between beliefs and TTR and longitudinal adherence in those who switch to a direct oral anticoagulant (DOAC).23 The primary aim of this study is to investigate differences in the beliefs between those with optimal and suboptimal TTR. A secondary objective is to investigate whether beliefs and clinical demographic variables are associated with TTR.
2. METHODS
This cross‐sectional observational study was conducted in the outpatient setting across the two anticoagulation clinics of King’s College Hospital NHS Foundation Trust in South East London. The Denmark Hill (DH) site is a tertiary center in a densely populated inner‐city area with a skew in the population towards lower socioeconomic status. The Princess Royal University Hospital (PRUH), situated between London and Kent, has an affluent catchment with a predominantly elderly Caucasian population.24
A random sample of patients prescribed VKAs for AF or secondary prevention of VTE with a TTR >75% over the previous year were identified using the DAWN (4S Information Systems, Ltd., Cumbria, England) databases at each site and sent a questionnaire pack with a patient information leaflet describing the study, a consent form, a prepaid envelope by which to return the completed questionnaire pack, and a complimentary tea bag. Patients with TTR <50%, were also identified via the DAWN database and invited to a pharmacist‐led consultation in clinic. For the TTR <50% group, patients were purposively sampled, the questionnaire pack was sent by mail accompanied by a clinic appointment letter. Questionnaires were to be completed at baseline prior to their appointment where they were counselled and offered the opportunity to change anticoagulation therapy to a DOAC provided it was clinically appropriate to do so. Patients who were switched to a DOAC for an unlicensed indication were done so under the instruction and supervision of a consultant hematologist. Patients were not incentivized to complete the questionnaire prior to their consultation and were made aware that participation in the study would not impact subsequent treatment decisions. TTR was calculated by the Rosendaal method and was measured over the previous 12 months. For those who had been prescribed VKAs for less than 12 months, the TTR over that entire period was used, provided they had been prescribed VKAs for more than 3 months.
Cut‐offs of TTR <50% and TTR >75% were selected as a binary variable was required for logistic regression. The cut off for “good” control is typically set at 65%.2, 8 As TTR is dynamic, extremes were opted for to determine clear differences between those with optimal control and those with poor control. Furthermore, research has demonstrated that compared to the poorly controlled cohort with a median TTR of 50%, those with a TTR >75% were far less likely to suffer a major event (HR = 0.164, P < .05).25 As such, the decided cut‐offs were deemed both clinically appropriate and statistically necessary.
To be eligible for the study, patients needed to be: aged over 18, prescribed lifelong anticoagulation, VKA treatment duration greater than 12 weeks, capable of providing informed consent, and able to read English. Patients with active cancer, autoimmune disease, and metallic heart valves were excluded from the study.
Clinical and demographic data was collected and CHA2DS2VASc, CHADS2, HAS‐BLED, Charlson comorbidity index (CCI), and SAMe‐TT₂R scores calculated.26, 27, 28, 29, 30 As the HAS‐BLED scoring system for determining bleeding risk allocates 1 point for TTR <60% which is also the grouping variable, true HAS‐BLED and adjusted HAS‐BLED scores were calculated, where the adjusted HAS‐BLED score discounts the point allocated for TTR <60%.
2.1. Study tools
Illness beliefs, medication beliefs, and treatment satisfaction/anticoagulation‐specific quality of life were assessed using a questionnaire pack comprised of the Revised Illness Perceptions Questionnaire (IPQ‐R), the Beliefs about Medicines Questionnaire (BMQ), and the Anti‐Clot Treatment Scale (ACTS), respectively.31, 32, 33 Questions in the IPQ‐R and BMQ are answered on a 5‐point Likert scale “strongly disagree” ascending to “strongly agree” which are scored as 1 and 5, respectively. ACTS is answered on a 5‐point Likert scale ranging from “not at all” to “extremely” scored as 1 and 5, respectively.
The IPQ‐R and BMQ are derived from Leventhal’s Common Sense Model of Health and Illness (CSM) which explains that when confronted with health threats, coping mechanisms deconstruct the threat into various illness representations: identity, timeline acute‐chronic, timeline cyclical, consequences, personal control, treatment control, cause, illness control, and emotional distress.31 The BMQ assesses beliefs regarding medication on four subscales; general harm and general overuse explore beliefs surrounding medication in general. Specific necessity determines the extent the patient recognises the need for VKA therapy, while the specific concern subscale determines how strong their anxieties towards VKAs are.32 The ACTS measures anticoagulation‐specific QoL and treatment satisfaction on two subscales: benefits and burdens of anticoagulation therapy.33, 34 For descriptions of all subscales see Table 1. The Cronbach’s alpha test for internal consistency was used on each of the questionnaire subscales where an alpha score >0.6 is considered reliable.35 All subscales had Cronbach’s α scores >0.6 other than treatment control where α = 0.407 and accidental cause α = 0.170, the latter being consistent with the validation study.31 See Supplementary Table S1.
Table 1.
Subscale Description and Scoring
| Questionnaire | Subscale | Description | Minimum score | Maximum score |
|---|---|---|---|---|
| IPQ‐R | Timeline Acute Chronic | Patient’s perception of disease duration | 6 | 30 |
| Timeline Cyclical | Patient perception that disease will come and go | 4 | 20 | |
| Consequences | Patient perception of disease impact | 6 | 30 | |
| Personal Control | Extent to which patient believes they can impact disease outcome, i.e., self‐efficacy | 6 | 30 | |
| Treatment Control | Extent to which patient believes treatment will be able to manage disease | 6 | 30 | |
| Illness Coherence | Patient self‐reported understanding of illness | 5 | 25 | |
| Emotional Response | Emotional response evoked by illness | 6 | 30 | |
| BMQ | General Harm | Extent to which patient believes any medication is harmful | 4 | 20 |
| General Overuse | Extent to which patient believes medicines are overused in health care | 4 | 20 | |
| Specific Concern | Patient anticoagulation specific concerns | 5 | 25 | |
| Specific Necessity | Patient’s perceived need for anticoagulation therapy | 5 | 25 | |
| ACTS | Burdens | Anticoagulation‐specific impediments to quality of life | 12 | 60 |
| Benefits | Patient‐reported gains from anticoagulation therapy | 3 | 15 |
ACTS, Anti‐Clot Treatment Scale; BMQ, Beliefs about Medicines Questionnaire; IPQ‐R, Revised Illness Perception Questionnaire.
2.2. Statistical analysis
Data from completed questionnaires were analyzed using Statistical Package for the Social Sciences (SPSS) version 24, (IBM Corp., Armonk, NY). Descriptive statistics were used to describe clinical and demographic variables. Chi‐squared tests were used to compare nominal variables. Continuous variables were assessed for normality and independent t tests were conducted to compare continuous variables including subscale scores. Non‐normally distributed subscales would be compared using Wilcoxon‐rank tests. Necessity‐concerns differentials (NCDs) were calculated by subtracting the specific concerns subscale score from the specific necessity subscale score, where positive differentials indicate that necessity beliefs outweigh concerns.36 All tests were 2‐tailed with significance set at P ≤ .05.
Cluster analysis was performed on subscale scores to profile patients according to beliefs, other than causes subscales. Cluster analysis reduces the number of variables and groups subjects according to the entirety of their beliefs. Hierarchical cluster analysis using Ward’s method with squared Euclidean distance for pairing of subjects was used. One‐way analysis of variance (ANOVA) was undertaken to determine differences in subscale scores between clusters. Assuming unequal homogeneity of variance, the Games‐Howell post hoc test was used to establish differences between individual clusters. Descriptive characteristics of each cluster’s subscale scores were calculated by separating the mean subscale score for the cluster into four equal quartiles from high to low. To determine any association between beliefs and TTR category, univariate binary logistic regression was performed to establish which variables would be entered into a multivariate binary logistic regression model. Variables with P ≤ .1 at the univariate stage were entered into a backwards elimination logistic regression multivariate analysis modelling for TTR <50% (see supplementary materials for variables). Continuous variables were tested for linearity of the logit prior to this stage, variables that did not pass this test were excluded from the multivariate stage. Bootstrapping was performed in the independent t tests and regression analyses to eliminate bias from unequal sample sizes in each group.
Each subscale had a mean score calculated to account for any missing answers. Subscales with six items were allowed a maximum of two missing answers while subscales with less than six items were allowed a maximum of one missing answer to be included in the final analysis. The ACTS burdens subscale required a minimum of 9 out of 12 answered questions to be included in analysis. TTR <50% patients who returned incomplete questionnaires were asked to complete the missed answers following the consultation and providing consent. TTR >75% patients were not approached to complete incomplete questionnaires.
2.3. Power calculation
Assuming a recruitment ratio of 1:1 of TTR >75%: TTR <50%, a total sample size of 180 to 240 patients would be able to accommodate 12 predictive variables for binary logistic regression; i.e., up to 120 patients with TTR >75% and up to 120 patients with TTR <50%. As TTR <50% patients would enter a longitudinal study which had a recruitment target of 240, a recruitment ratio of 1:1 was maintained for this study.
2.4. Ethical approval
This study was reviewed and approved by the London‐Dulwich Research Ethics Committee (13/LO/1468) and King’s College Hospital NHS Foundation Trust research and development (KCH14‐111).
3. RESULTS
Between September 2014 and October 2016, 525 patients were recruited. Of 1049 questionnaires mailed to TTR >75% patients, 326 (31%) were returned and 15 of these were incomplete and not used. The identity section of IPQ‐R was consistently poorly answered with many patients leaving several questions blank, therefore this section of the IPQ‐R was not analyzed. Patients in TTR >75% were older and had been prescribed VKAs for longer than those with TTR <50% across all disease groups (P < .001 for all), see Table 2. Among AF patients there were no differences in stroke risk as calculated by either CHADS2 or CHA2DS2VASc between groups, nor was there any difference in adjusted HAS‐BLED score between groups.
Table 2.
Clinical and Demographic Information of Recruited Patients
| AF patients | VTE patients | Other diagnosesa , b | ||||||
|---|---|---|---|---|---|---|---|---|
| TTR >75% (n = 187) | TTR <50% (n = 164) | p value | TTR >75% (n = 89) | TTR <50% (n = 43) | p value | TTR >75% (n = 35) | TTR <50% (n = 7) | |
| TTR (median, IQR) | 90 (81‐100) | 39 (33‐45) | — | 95 (87‐100) | 38 (32‐44) | — | 97 (89‐100) | 35 (34‐36) |
| KCHc (n, %) | 86 (46) | 66 (40) | 0.278 | 41 (46) | 23 (53) | 0.424 | 19 (54) | 3 (43) |
| Male (n, %) | 112 (60) | 105 (64) | 0.427 | 53 (60) | 24 (56) | 0.693 | 26 (74) | 4 (47) |
| Caucasian (n, %) | 175 (94) | 143 (90) | 0.215 | 77 (87) | 31 (72) | 0.044 | 32 (91) | 7 (100) |
| Age (median, IQR) | 78 (71‐84) | 74 (66‐81) | <0.001 | 69 (61‐77) | 54 (46‐66) | <0.001 | 72 (63‐79) | 53 (50‐68) |
| Duration of Warfarin Therapy in weeks (median, IQR) | 221 (129‐352) | 116 (41‐275) | <0.001 | 269 (127‐595) | 139 (78‐260) | <0.001 | 299 (154‐610) | 194 (133‐198) |
| CHADS2 (mean, SD)d | 2.22 (1.23) | 2.15 (1.26) | 0.601 | N/A | NA | |||
| CHA2DS2VASc (mean, SD)e | 3.61 (1.44) | 3.37 (1.45) | 0.130 | N/A | NA | |||
| HAS‐BLED (mean, SD)f | 1.47 (0.67) | 2.44 (0.85) | <0.001 | 0.98 (0.75) | 1.74 (0.66) | <0.001 | 1.67 (1.04) | 2.14 (1.35) |
| Adjusted HAS‐BLED (mean, SD) | 1.47 (0.67) | 1.44 (0.85) | 0.682 | 0.98 (0.75) | 0.74 (0.66) | <0.087 | 1.67 (1.04) | 1.14 (1.35) |
| SAMe‐TT2R2 g (mean, SD) | 1.24 (0.89) | 1.48 (1.11) | 0.035 | 1.17 (1.11) | 2.26 (1.16) | <0.001 | 1.11 (0.94) | 2.86 (1.07) |
| CCI 10 year mortality (mean, SD)h | 0.27 (0.26) | 0.21 (0.29) | 0.057 | 0.47 (0.33) | 0.60 (0.39) | 0.066 | 0.43 (0.29) | 0.28 (0.28) |
| Congestive Heart Failure (n, %) | 51 (30) | 51 (31) | 0.828 | 8 (9) | 1 (2) | 0.143 | 6 (22) | 6 (86) |
| Hypertension (n, %) | 106 (62) | 99 (60) | 0.709 | 40 (47) | 14 (33) | 0.130 | 13 (48) | 2 (29) |
| Stroke (n, %) | 38 (22) | 36 (22) | 0.930 | 1 (1) | 2 (5) | 0.215 | 7 (26) | 5 (71) |
| Vascular Disease (n, %) | 13 (8) | 10 (6) | 0.576 | 4 (5) | 3 (7) | 0.583 | 1 (4) | 0 (0) |
| Type II Diabetes Mellitus (n, %) | 28 (16) | 41 (25) | 0.054 | 14 (16) | 6 (14) | 0.731 | 6 (22) | 1 (14) |
| Bleeding History (n, %) | 21 (12) | 20 (12) | 0.965 | 7 (8) | 3 (7) | 0.816 | 5 (19) | 1 (14) |
| Myocardial Infarction (n, %) | 28 (16) | 16 (10) | 0.073 | 3 (3) | 3 (7) | 0.375 | 7 (26) | 3 (43) |
| Ischaemic Heart Disease (n, %) | 46 (27) | 46 (28) | 0.840 | 6 (7) | 3 (7) | 1.000 | 9 (33) | 3 (43) |
Aortic valve replacement, mitral valve replacement, left ventricular thrombus.
Due to small number of participants in this group, statistical tests not performed.
King’s College Hospital Clinic Site.
CHADS2: congestive heart failure, hypertension, diabetes and age >75 years are assigned 1 point, prior stroke/TIA assigned 2 points.
CHA2DS2VASc: congestive heart failure, hypertension, diabetes, female gender, vascular disease and an age of 65‐74 years assigned 1 point, prior stroke/TIA and age >75 years assigned 2 points.
HAS‐BLED: uncontrolled hypertension, severe renal impairment, liver disease, stroke, bleeding history, TTR <60%, age >65 years, drug increasing bleeding risk, alcohol consumption >8 drinks/wk.
SAMe‐TT2R2: female gender, age <60 years, 2 of: hypertension, diabetes, peripheral artery disease, congestive heart failure, stroke, respiratory disease, liver disease, renal disease, myocardial infarction, interacting treatment assigned 1 point, tobacco use in previous 2 years and non‐Caucasian race assigned 2 points.
Charlson Co‐Morbidity Index Predicting likelihood of 10 year mortality.
3.1. Differences in beliefs
Amongst AF patients, those with TTR >75% had stronger chronic timeline beliefs and were less likely to believe that their illness was cyclical (Table 3). TTR <50% patients had greater concern about the consequence of their illness while also experiencing greater emotional distress because of their AF. TTR <50% patients reported believing more strongly in the potential for harm from pharmaceutical products accompanied by greater concerns regarding VKAs specifically, whilst perceiving it to be less necessary. According to both the benefits and burdens subscales, TTR <50% patients had lower treatment satisfaction.
Table 3.
Independent T‐tests for Subscales Measured
| Questionnaire | Subscale | AF patients | VTE patients | ||||
|---|---|---|---|---|---|---|---|
| TTR >75%(n = 187)Mean (SD) | TTR <50%(n = 164)Mean (SD) | p value | TTR >75%(n = 89)Mean (SD) | TTR <50%(n = 43)Mean (SD) | p value | ||
| IPQ‐R | Timeline Acute Chronic | 24.3 (3.8) | 23.1 (4.1) | 0.008 | 25.2 (4.3) | 23.3 (5.0) | 0.015 |
| Timeline Cyclical | 8.9 (3.1) | 10.4 (3.2) | 0.001 | 8.0 (3.3) | 10.3 (3.7) | 0.002 | |
| Consequences | 16.2 (3.9) | 17.6 (4.4) | 0.003 | 16.8 (4.1) | 18.4 (4.5) | 0.037 | |
| Personal Control | 18.8 (4.4) | 19.7 (4.0) | 0.089 | 18.4 (4.8) | 19.8 (4.0) | 0.141 | |
| Treatment Control | 16.4 (2.3) | 16.9 (2.6) | 0.074 | 17.1 (2.4) | 17.1 (3.0) | 0.898 | |
| Illness Coherence | 18.5 (3.9) | 18.3 (3.9) | 0.446 | 18.9 (4.0) | 17.2 (4.4) | 0.027 | |
| Emotional Response | 14.2 (4.4) | 15.3 (4.7) | 0.006 | 14.5 (5.4) | 15.8 (5.1) | 0.230 | |
| Psychological Cause | 13.4 (4.1) | 13.4 (4.0) | 0.533 | 11.4 (3.9) | 13.0 (4.5) | 0.067 | |
| Risk Factor Cause | 17.0 (3.9) | 17.8 (4.2) | 0.067 | 15.3 (3.8) | 16.5 (5.1) | 0.255 | |
| Immune Cause | 6.4 (1.9) | 6.8 (1.7) | 0.017 | 6.0 (2.1) | 6.9 (2.5) | 0.041 | |
| Accidental Cause | 4.5 (1.5) | 4.6 (1.5) | 0.142 | 5.2 (1.7) | 5.6 (1.8) | 0.184 | |
| BMQ | General Harm | 19.9 (6.5) | 23.5 (8.0) | 0.044 | 8.9 (2.4) | 9.7 (2.9) | 0.141 |
| General Overuse | 11.4 (2.7) | 10.1 (2.7) | 0.075 | 11.2 (2.7) | 11.8 (3.3) | 0.281 | |
| Specific Concern | 12.0 (3.5) | 13.9 (3.7) | 0.001 | 13.0 (3.6) | 14.6 (4.4) | 0.032 | |
| Specific Necessity | 17.3 (3.7) | 16.5 (3.2) | 0.017 | 18.5 (3.6) | 17.8 (3.8) | 0.196 | |
| ACTS | Burdens | 19.9 (6.5) | 23.5 (8.0) | 0.001 | 23.5 (8.3) | 26.3 (9.3) | 0.093 |
| Benefits | 11.4 (2.7) | 10.1 (2.7) | 0.001 | 11.7 (2.9) | 11.6 (2.4) | 0.721 | |
For VTE patients, illness beliefs were broadly similar to AF patients with some key exceptions: TTR <50% patients have lower illness coherence and do not experience different levels of emotional distress. While TTR <50% VTE patients have greater concerns about VKAs, both groups have similar beliefs regarding the necessity of anticoagulation treatment. There were no differences in treatment satisfaction.
3.2. Patterns of beliefs
Cluster analysis revealed four distinct belief sets (Table 4). Cluster 1 were accepting of both their therapy and their illness, cluster 2 was cautious of therapy but accepting of their illness, cluster 3 was accepting of therapy but fearful about their illness, while cluster 4 was cautious of therapy and fearful of their disease. Clinical demographics according to cluster are shown in Table 5. Cluster 1 was the most populous where patients were likely to be well controlled with VKAs. Over 65% of patients in cluster 4 were poorly controlled on VKAs and over 50% of patients in this cluster were female compared to approximately 35% in all other clusters. Post hoc analysis of subscale scores showed significant differences between clusters in every subscale, validating the cluster analysis, see Supplementary Table S2.
Table 4.
Cluster Characteristics
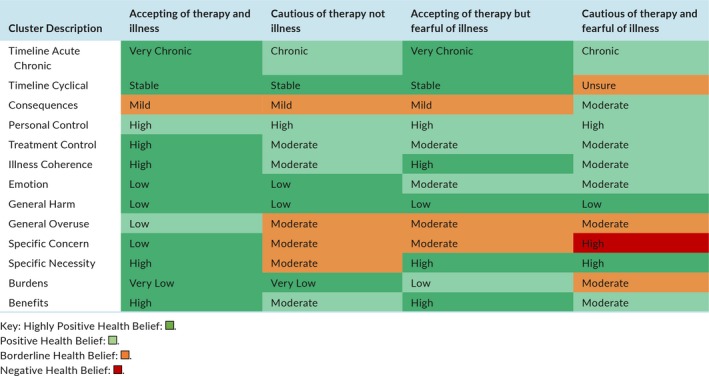
Table 5.
Clinical and Demographic Characteristics of Clusters
| Cluster 1 (n = 186) | Cluster 2 (n = 92) | Cluster 3 (n = 120) | Cluster 4 (n = 76) | |
|---|---|---|---|---|
| Age (median, IQR) | 73 (66‐81) | 77 (69‐84) | 72 (62‐78) | 70 (61‐77) |
| TTR >75% (n, %) | 132 (71.0) | 47 (51.1) | 64 (53.3) | 28 (36.8) |
| Male (n, %) | 121 (65.1) | 60 (65.2) | 78 (65.0) | 37 (48.7) |
| Caucasian (n, %) | 180 (96.8) | 79 (87.8) | 103 (86.6) | 61 (81.3) |
| AF (n, %) | 127 (68.3) | 68 (73.9) | 75 (62.5) | 47 (61.8) |
| VTE (n, %) | 43 (23.1) | 21 (22.8) | 34 (28.3) | 22 (29.0) |
| Other (n, %) | 16 (8.6) | 3 (3.3) | 11 (9.2) | 7 (9.2) |
| CHADS2 (mean, SD)a | 2.1 (1.2) | 2.3 (1.4) | 2.3 (1.4) | 2.0 (1.0) |
| CHA2DS2VASc (mean, SD)a | 3.4 (1.3) | 3.6 (1.6) | 3.4 (1.6) | 3.4 (1.2) |
| HAS‐BLED (mean, SD) | 1.7 (1.0) | 1.9 (0.9) | 1.8 (0.9) | 1.8 (0.9) |
| CCI 10 year mortality (mean, SD) | 0.4 (0.3) | 0.3 (0.3) | 0.3 (0.3) | 0.3 (0.3) |
| SAME‐TTR (mean, SD) | 1.1 (0.9) | 1.4 (1.1) | 1.4 (1.1) | 1.9 (1.2) |
AF patients only.
3.3. Factors influencing TTR
Variables linked with low TTR according to univariate logistic regression with P ≤ .1 that were subsequently entered into a multivariate model determining TTR <50% were: ethnicity, diagnosis, drug increasing bleed risk, thrombotic history, belief cluster, and concurrent ACE‐ inhibitor prescription. The reference category for nominal variables were: Caucasian ethnicity, diagnosis of AF, not being prescribed an ACE‐inhibitor or drug increasing bleeding risk, no thrombotic history, and belief cluster 1. The resulting model found that only diagnosis and belief cluster were associated with TTR <50% (Table 6). VTE patients were more than half as likely to have poor INR control (OR = 0.49; 95% CI 0.29, 0.77) while there was increasing risk moving from cluster 1 to 4 where cluster 4 patients were over four times more likely to be poorly controlled (OR = 4.75; 95% CI 2.75, 8.77). This model describes 65% of the variance in TTR category. Age, gender, income, life expectancey, and disease severity were not associated with TTR according to regression analysis, and thus the model was not adjusted for variables such as age or gender (see supplementary information).
Table 6.
Unadjusted Binary Logistic Regression Model Predicting TTR <50%
| Odds ratio (OR) | 95% C.I. for OR | ||
|---|---|---|---|
| Lower | Upper | ||
| AF (Ref) | 1.00 | — | — |
| VTE | 0.49 | 0.29 | 0.77 |
| Other | 0.22 | 0.07 | 0.46 |
| Cluster 1 (Ref) | 1.00 | — | — |
| Cluster 2 | 2.25 | 1.35 | 3.77 |
| Cluster 3 | 2.32 | 1.42 | 3.74 |
| Cluster 4 | 4.75 | 2.75 | 8.77 |
Diagnosis of AF and Cluster 1 were baseline comparators.
3.4. AF versus VTE
Comparing AF and VTE patients with TTR >75% (Table 7) revealed VTE patients have greater concerns about therapy, necessity beliefs, and burdens compared to AF patients. These VTE patients also had greater conviction in their illness being caused by accident or bad luck. AF patients with TTR >75% were more likely to attribute their illness to psychological causes such as stress, family problems, or overwork. AF patients were also more likely to believe that their illness was caused by risk factors such as genetics, unhealthy eating, and poor medical care.
Table 7.
Independent T‐Tests Comparing AF and VTE Patients Within TTR Group
| Questionnaire | Subscale | TTR >75% | TTR <50% | ||||
|---|---|---|---|---|---|---|---|
| AF(n = 187)Mean (SD) | VTE(n = 89)Mean (SD) | p value | AF(n = 164)Mean (SD) | VTE(n = 43)Mean (SD) | p value | ||
| IPQ‐R | Timeline Acute Chronic | 24.3 (3.8) | 25.2 (4.3) | 0.092 | 23.1 (4.1) | 23.3 (5.0) | 0.889 |
| Timeline Cyclical | 8.9 (3.1) | 8.0 (3.3) | 0.200 | 10.4 (3.2) | 10.3 (3.7) | 0.724 | |
| Consequences | 16.2 (3.9) | 16.8 (4.1) | 0.283 | 17.6 (4.4) | 18.4 (4.5) | 0.374 | |
| Personal Control | 18.8 (4.4) | 18.4 (4.8) | 0.595 | 19.7 (4.0) | 19.8 (4.0) | 0.927 | |
| Treatment Control | 16.4 (2.3) | 17.1 (2.4) | 0.075 | 16.9 (2.6) | 17.1 (3.0) | 0.915 | |
| Illness Coherence | 18.5 (3.9) | 18.9 (4.0) | 0.529 | 18.3 (3.9) | 17.2 (4.4) | 0.119 | |
| Emotional Response | 14.2 (4.4) | 14.5 (5.4) | 0.337 | 15.3 (4.7) | 15.8 (5.1) | 0.586 | |
| Psychological Cause | 13.4 (4.1) | 11.4 (3.9) | 0.001 | 13.4 (4.0) | 13.0 (4.5) | 0.245 | |
| Risk Factor Cause | 17.0 (3.9) | 15.3 (3.8) | 0.001 | 17.8 (4.2) | 16.5 (5.1) | 0.051 | |
| Immune Cause | 6.4 (1.9) | 6.0 (2.1) | 0.135 | 6.8 (1.7) | 6.9 (2.5) | 0.999 | |
| Accidental Cause | 4.5 (1.5) | 5.2 (1.7) | 0.004 | 4.6 (1.5) | 5.6 (1.8) | 0.003 | |
| BMQ | General Harm | 19.9 (6.5) | 8.9 (2.4) | 0.097 | 23.5 (8.0) | 9.7 (2.9) | 0.074 |
| General Overuse | 11.4 (2.7) | 11.2 (2.7) | 0.478 | 10.1 (2.7) | 11.8 (3.3) | 0.500 | |
| Specific Concern | 12.0 (3.5) | 13.0 (3.6) | 0.020 | 13.9 (3.7) | 14.6 (4.4) | 0.340 | |
| Specific Necessity | 17.3 (3.7) | 18.5 (3.6) | 0.012 | 16.5 (3.2) | 17.8 (3.8) | 0.042 | |
| ACTS | Burdens | 19.9 (6.5) | 23.5 (8.3) | 0.001 | 23.5 (8.0) | 26.3 (9.3) | 0.069 |
| Benefits | 11.4 (2.7) | 11.7 (2.9) | 0.704 | 10.1 (2.7) | 11.6 (2.4) | 0.004 | |
Comparing AF and VTE patients with TTR <50% demonstrated that VTE patients had greater specific necessity beliefs relating to VKAs and were more likely to recognize the benefits of therapy than AF patients. Although compared to AF patients, VTE patients reported greater burdens of VKA therapy, this was not statistically significant. Like those with TTR >75%, VTE patients in this group attributed their illness to accident or bad luck more frequently than AF patients. There were no significant differences in NCDs between AF and VTE in either optimal or suboptimal groups (Supplementary Tables 3 and 4).
4. DISCUSSION
This study has demonstrated how illness beliefs, medication beliefs, and anticoagulation‐specific QoL and treatment satisfaction differ between those with optimal (TTR >75%) and suboptimal (TTR <50%) INR control. To our knowledge, this is the first study designed to examine the differing beliefs between these groups and to determine belief patterns associated with TTR. The differences in beliefs were largely similar for both the AF and VTE patients, albeit with some exceptions. In examining the differences between the two groups, there are two key aspects to this VKA experience: understanding and knowledge of illness and subjective experiences of their illness and treatment.
4.1. Understanding and knowledge of illness
Despite all patients being prescribed VKAs lifelong, timeline beliefs were markedly different between groups. Those with TTR >75% with either condition believed more strongly in the chronic and noncyclical nature of their illness. This accompanied by lower illness coherence among TTR <50% VTE patients, highlights a knowledge deficit among patients with TTR <50% about the course and nature of their illness.
Among AF patients, those with TTR <50% believed less in VKA necessity, indicating these patients may be less aware of the risk of stroke associated with AF and the role anticoagulation plays in preventing it. Conversely, VTE patients had similar necessity beliefs across the board. This is likely to be related to VKA therapy being the active treatment used for acute VTE and therefore they placed a higher value on VKA treatment for secondary prevention. Research elsewhere has demonstrated that a strong sense of necessity, is linked to better adherence while strong concerns have the opposite effect.37 In practice, clinicians should elicit these beliefs at the outset to ensure appropriate understanding. Although interventions in anticoagulation that have targeted education alone have had limited success,38, 39 there is scope to improve TTR by addressing inaccurate beliefs if combined with other behavior change techniques. Interventions utilising nonmedical personnel such as pharmacists and nurses have proved to be successful.40
4.2. Subjective experiences of illness and treatment
AF patients with TTR <50% reported fewer benefits of anticoagulation and greater emotional distress, specific concerns and burdens of therapy, while TTR <50% VTE patients scored higher in specific concerns and trended towards greater burdens. This is indicative of negative experiences regarding emotional wellbeing and treatment satisfaction. This is consistent with Lane et al’s work which found that high baseline specific concern was consistent with worse QoL.41 It is likely that the experience of out‐of‐range INR results, dose changes, and more frequent testing has perpetuated negative perceptions. In conjunction with this, interactions from the clinic including phone calls enquiring about blood loss and bruising may contribute to the patient’s raised risk perception as demonstrated by TTR <50% patients’ strong sense that their illness has severe consequences.42
TTR >75% patients were prescribed VKAs on average nearly 2 years longer than TTR <50% patients. This may explain some of the differences as optimal‐TTR patients had longer to establish beliefs and behaviors around their VKAs. However, duration of VKA therapy was not associated with TTR category in regression analyses. Furthermore, it is to be expected that patients with TTR <50% have a shorter duration of therapy as guidelines dictate that these patients should be considered for switching to DOAC.2, 8
4.3. Belief patterns associated with TTR
Cluster analysis revealed four distinct patterns of beliefs among patients ranging from cluster 1, who were accepting of both their therapy and illness, to those who were fearful of both, i.e, cluster 4. Regression analysis found patients at the highest risk of having poor INR control were those with AF and beliefs akin to cluster 4. Although cluster 4 was made up of disproportionately more women, gender was unrelated to TTR in the regression analysis and is not consistently related to adherence or TTR in the literature.22
VTE patients were less likely to have low TTR. This may be due to previous experience of thrombosis where VKAs were the active treatment. Referring to the COM‐B model, motivation to adhere would be enhanced, while enhancing necessity perceptions. The TREAT study found that baseline specific necessity beliefs were predictive of INR control at 1 year. Furthermore, they established links between baseline negative beliefs about medication and TTR.43 This finding is supported by our analysis where TTR >75% patients have higher necessity beliefs. AF patients with a history of stroke did not report higher emotional distress or specific necessity than those without stroke, although the number of stroke patients was relatively small. Stroke was unrelated to TTR in the regression analysis.
The regression analysis has been reported unadjusted for age, gender, or any other clinical‐demographic variable. The development of this model was exploratory and all variables underwent univariate logistic regression to determine any association with TTR, as no clinical‐demographic variable influenced TTR, none was adjusted for in the final multivariate model. Within the literature, there is no strong evidence that any clinical demographic is associated with adherence to anticoagulation or TTR that would provide a basis by which to adjust for them,22, 44 nor is there a theoretical basis to do so.
4.4. Beliefs of AF and VTE patients
TTR >75% VTE patients had a greater recognition of the necessity for VKA therapy compared to those with AF, similar to findings in Dutch patients.45 Unexpectedly, our VTE patients had greater concerns and reported more burdens related to VKAs. The NCDs between AF and VTE patients were similar in both groups. This is in contrast to previous research, which has found that VTE patients had higher differentials compared to AF patients.45 A possible explanation for this is our VTE patients were on average 10 years younger and younger age has been associated with worse adherence elsewhere.46, 47 Furthermore, as the AF population was more comorbid, VTE patients were likely to recognize the burden of their illness more than AF patients where AF is one of many illnesses. Beyond this, although not statistically significant, VTE patients exhibited stronger consequence beliefs regarding their illness. As all VTE patients had experienced a symptomatic event previously, this heightened risk perception compared to AF patients is intuitive. Kaptein et al previously reported that among those at high risk of thrombosis, those who had previous experience of a thrombus had significantly higher risk perception.48 This could explain the raised necessity and consequence perceptions in VTE.
4.5. Validity of study tools
The high internal consistency through Cronbach’s alpha validates the use of the IPQ‐R, BMQ, and ACTS for use in AF and VTE patients prescribed VKAs.35 The exceptions were the accidental cause of the treatment control subscales. The latter is mitigated through the analogous subscales from the BMQ and ACTS questionnaires and was not improved by removing any one question from the subscale. The alpha score for accidental cause is similar to that of the validation study for IPQ‐R and is low due to there being only two questions in the subscale.31
4.6. Implications for practice
This research has demonstrated that beliefs vary significantly with TTR. Furthermore, the regression analysis has shown that beliefs are associated with TTR. Although potentially useful for screening purposes, clinical demographic variables such as age, gender, disease severity, duration of warfarin treatment, comorbidities, and income were not associated with TTR. Even if they were, these variables are nonmodifiable. Crucially, beliefs can be modified. Beliefs that are prevalent among those with low TTR could potentially be targeted to initiate behavior change with the goal of improving TTR and adherence. In the age of DOACs, where the consensus is that patients with poor TTR should be considered for DOAC treatment, these belief patterns still need to be addressed and can act as a valuable tool in effective patient management.
4.7. Limitations
While low TTR is associated with poor adherence to VKAs, it is not the only cause. Other factors include dietary intake, other medication, genetic polymorphism, or inappropriate VKA dosing. This is a noninterventional study and all TTR <50% patients were asked to complete their questionnaire prior to consultation to prevent bias; however, some incomplete questionnaires were submitted. In this case patients were asked to be complete missing answers after the consultation, potentially affecting the results in a small number of patients. This study also does not provide insight into beliefs about VKAs in the context of the patient’s other medication. Due to the cross‐sectional nature of this study, the beliefs of patients with TTR between 51% and 74% are unknown.
5. CONCLUSIONS
This study has demonstrated that illness beliefs, medication beliefs, and QoL in patients prescribed chronic VKA therapy are significantly associated with the behavior of anticoagulated patients and are associated with INR control. The multiple disparities between those with TTR >75% and TTR <50% groups can be targeted through theory‐driven interventions to attempt improving TTR and support medication adherence.
RELATIONSHIP DISCLOSURES
Professor Arya has received honoraria for lectures and travel from Bayer, Boehringer Ingelheim, and Pfizer, and awards for investigator‐sponsored research from Bayer and Covidien. Dr Auyeung and Dr J.P. Patel have received investigator initiated research funding from Bayer. Dr Vadher has received travel grants and event sponsorship from Boehringer‐Ingelheim and Bayer. Dr Roberts has received speaker fees from Bayer. Dr R. Patel has received honoraria from Bayer, Boehringer‐Ingelheim, and BMS‐Pfizer. Alison Brown has received a travel grant from Daiichi Sankyo. John Bartoli‐Abdou, Olubanke Dzahini, and Rosa Xie have no disclosures to declare.
AUTHOR CONTRIBUTIONS
This manuscript was drafted by JKBA, was subsequently revised by JPP and VA and reviewed by the other authors. OD provided oversight and expert assistance with statistical analysis.
Supporting information
Bartoli‐Abdou JK, Patel JP, Xie R, et al. Associations between illness beliefs, medication beliefs, anticoagulation‐related quality of life, and INR control: Insights from the Switching Study. Res Pract Thromb Haemost. 2018;2:497–507. 10.1002/rth2.12116
This research is funded by an investigator initiated research grant from Bayer PLC.
Contributor Information
John K. Bartoli‐Abdou, Email: john.abdou@kcl.ac.uk, https://twitter.com/adhere_anticoag.
Roopen Arya, https://twitter.com/AryaRoopen.
REFERENCES
- 1. Camm AJ, Lip GY, De Caterina R, et al.; ESC Committee for Practice Guidelines (CPG) . 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur Heart J. 2012;33:2719–47. [DOI] [PubMed] [Google Scholar]
- 2. NICE . Atrial Fibrillation: Management: Clinical Guideline [CG180]. London, UK: National Institute for Health and Care Excellence; 2014. [PubMed] [Google Scholar]
- 3. NICE . Venous Thromboembolic Diseases: Diagnosis, Management and Thrombophilia Testing: Clinical Guideline [CG144]. London, UK: National Institute for Health and Care Excellence; 2012. [PubMed] [Google Scholar]
- 4. Jaff MR, McMurtry MS, Archer SL, et al. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: a scientific statement from the American Heart Association. Circulation. 2011;123:1788–830. [DOI] [PubMed] [Google Scholar]
- 5. Di Minno A, Spadarella G, Tufano A, Prisco D, Di Minno G. Ensuring medication adherence with direct oral anticoagulant drugs: lessons from adherence with vitamin K antagonists (VKAs). Thromb Res. 2014;133:699–704. [DOI] [PubMed] [Google Scholar]
- 6. Ten Cate H. New oral anticoagulants: discussion on monitoring and adherence should start now!. Thromb J. 2013;11:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353:487–97. [DOI] [PubMed] [Google Scholar]
- 8. Heidbuchel H, Verhamme P, Alings M, et al. Updated European Heart Rhythm Association Practical Guide on the use of non‐vitamin K antagonist anticoagulants in patients with non‐valvular atrial fibrillation. Europace. 2015;17:1467–507. [DOI] [PubMed] [Google Scholar]
- 9. Rosendaal FR, Cannegieter SC, van der Meer FJ, Briet E. A method to determine the optimal intensity of oral anticoagulant therapy. Thromb Haemost. 1993;69:236–9. [PubMed] [Google Scholar]
- 10. Morgan CL, McEwan P, Tukiendorf A, Robinson PA, Clemens A, Plumb JM. Warfarin treatment in patients with atrial fibrillation: observing outcomes associated with varying levels of INR control. Thromb Res. 2009;124:37–41. [DOI] [PubMed] [Google Scholar]
- 11. Schein JR, White CM, Nelson WW, Kluger J, Mearns ES, Coleman CI. Vitamin K antagonist use: evidence of the difficulty of achieving and maintaining target INR range and subsequent consequences. Thromb J. 2016;14:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cruess DG, Localio AR, Platt AB, et al. Patient attitudinal and behavioral factors associated with warfarin non‐adherence at outpatient anticoagulation clinics. Int J Behav Med. 2010;17:33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sinauridze EI, Panteleev MA, Ataullakhanov FI. Anticoagulant therapy: basic principles, classic approaches and recent developments. Blood Coagul Fibrinolysis. 2012;23:482–93. [DOI] [PubMed] [Google Scholar]
- 14. Dantas GC, Thompson BV, Manson JA, Tracy CS, Upshur RE. Patients’ perspectives on taking warfarin: qualitative study in family practice. BMC Fam Pract. 2004;5:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. DiMatteo MR. Variations in patients’ adherence to medical recommendations: a quantitative review of 50 years of research. Med Care. 2004;42:200–9. [DOI] [PubMed] [Google Scholar]
- 16. World Health Organization . Adherence to Long‐Term Therapies: Evidence for Action. Geneva: World Health Organization; 2003: p. 110. [Google Scholar]
- 17. Horne R, Weinman J, Barber N, Elliot R, Morgan M. Concordance, adherence and compliance in medicine taking: Report for the National Co‐ordinating Centre for NHS Service Delivery and Organisation R&D (NCCSDO). Report for the National Co‐ordinating Centre for NHS Service Delivery and Organisation R&D (NCCSDO), London, UK, 2005:311. [Google Scholar]
- 18. Horne R. Treatment perceptions and self regulation In: Cameron LD, Leventhal H, eds. The Self‐Regulation of Health and Illness Behaviour. New York: Taylor & Francis, Inc; 2002; p. 138–53. [Google Scholar]
- 19. Jackson C, Eliasson L, Barber N, Weinman J. Applying COM‐B to medication adherence: a suggested framework for research and interventions. Eur Health Psychologist. 2014;16:7–17. [Google Scholar]
- 20. Leventhal H, Brissette I, Leventhal EA. The common sense model In: Cameron LD, Leventhal H, eds. The Self‐Regulation of Health and Illness Behaviour. New York: Taylor & Francis, Inc; 2003. [Google Scholar]
- 21. Michie S, van Stralen MM, West R. The behaviour change wheel: a new method for characterising and designing behaviour change interventions. Implement Sci. 2011;6:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Abdou JK, Auyeung V, Patel JP, Arya R. Adherence to long‐term anticoagulation treatment, what is known and what the future might hold. Br J Haematol. 2016;174:30–42. [DOI] [PubMed] [Google Scholar]
- 23. Auyeung V, Patel JP, Abdou JK, et al. Anticoagulated patient’s perception of their illness, their beliefs about the anticoagulant therapy prescribed and the relationship with adherence: impact of novel oral anticoagulant therapy—study protocol for The Switching Study: a prospective cohort study. BMC Hematology. 2016;16:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Greater London Authority . London Borough Profiles and AtlasSeptember 2015 edn. London, UK: GLA; 2015. [Google Scholar]
- 25. Pastori D, Pignatelli P, Saliola M, et al. Inadequate anticoagulation by Vitamin K antagonists is associated with major adverse cardiovascular events in patients with atrial fibrillation. Int J Cardiol. 2015;201:513–6. [DOI] [PubMed] [Google Scholar]
- 26. Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor‐based approach: the euro heart survey on atrial fibrillation. Chest. 2010;137:263–72. [DOI] [PubMed] [Google Scholar]
- 27. Gage BF, van Walraven C, Pearce L, et al. Selecting patients with atrial fibrillation for anticoagulation: stroke risk stratification in patients taking aspirin. Circulation. 2004;110:2287–92. [DOI] [PubMed] [Google Scholar]
- 28. Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user‐friendly score (HAS‐BLED) to assess 1‐year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest. 2010;138:1093–100. [DOI] [PubMed] [Google Scholar]
- 29. Poli D, Antonucci E, Testa S, Lip GY. A prospective validation of the SAME‐TT2R 2 score: how to identify atrial fibrillation patients who will have good anticoagulation control on warfarin. Intern Emerg Med. 2014;9:443–7. [DOI] [PubMed] [Google Scholar]
- 30. Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47:1245–51. [DOI] [PubMed] [Google Scholar]
- 31. Moss‐Morris R, Weinman J, Petrie K, Horne R, Cameron L, Buick D. The Revised Illness Perception Questionnaire (IPQ‐R). Psychol Health. 2002;17:1–16. [Google Scholar]
- 32. Horne R, Weinman J, Hankins M. The beliefs about medicines questionnaire: the development and evaluation of a new method for assessing the cognitive representation of medication. Psychol Health. 1999;14:1–24. [Google Scholar]
- 33. Cano SJ, Lamping DL, Bamber L, Smith S. The Anti‐Clot Treatment Scale (ACTS) in clinical trials: cross‐cultural validation in venous thromboembolism patients. Health Qual Life Outcomes. 2012;10:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Prins MH, Bamber L, Cano SJ, et al. Patient‐reported treatment satisfaction with oral rivaroxaban versus standard therapy in the treatment of pulmonary embolism; results from the EINSTEIN PE trial. Thromb Res. 2015;135:281–8. [DOI] [PubMed] [Google Scholar]
- 35. Tavakol M, Dennick R. Making sense of Cronbach’s alpha. Int J Med Educ. 2011;2:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Horne R, Weinman J. Patients’ beliefs about prescribed medicines and their role in adherence to treatment in chronic physical illness. J Psychosom Res. 1999;47:555–67. [DOI] [PubMed] [Google Scholar]
- 37. Horne R, Chapman SCE, Parham R, Freemantle N, Forbes A, Cooper V. Understanding patients adherence‐related beliefs about medicines prescribed for long‐term conditions: a meta‐analytic review of the necessity‐concerns framework. PLoS ONE. 2013;8:e80633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Clarkesmith DE, Pattison HM, Khaing PH, Lane DA. Educational and behavioural interventions for anticoagulant therapy in patients with atrial fibrillation. Cochrane Database Syst Rev. 2017;(4):CD008600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. van Dulmen S, Sluijs E, van Dijk L, de Ridder D, Heerdink R, Bensing J. Patient adherence to medical treatment: a review of reviews. BMC Health Serv Res. 2007;7:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wofford JL, Wells MD, Singh S. Best strategies for patient education about anticoagulation with warfarin: a systematic review. BMC Health Serv Res. 2008;8:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lane DA, Langman CM, Lip GY, Nouwen A. Illness perceptions, affective response, and health‐related quality of life in patients with atrial fibrillation. J Psychosom Res. 2009;66:203–10. [DOI] [PubMed] [Google Scholar]
- 42. Bartoli‐ Abdou JK, Patel JP, Crawshaw J, et al. Exploration of adherence and patient experiences with DOACs one year after switching from vitamin‐K antagonists‐ insights from the switching study. Thromb Res. 2018;162:62–8. [DOI] [PubMed] [Google Scholar]
- 43. Clarkesmith DE, Pattison HM, Lip GY, Lane DA. Educational intervention improves anticoagulation control in atrial fibrillation patients: the TREAT randomised trial. PLoS ONE. 2013;8:e74037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Raparelli V, Proietti M, Cangemi R, Lip GYH, Lane DA, Basili S. Adherence to oral anticoagulant therapy in patients with atrial fibrillation. Focus on non‐vitamin K antagonist oral anticoagulants. Thromb Haemost. 2017;117:209–18. [DOI] [PubMed] [Google Scholar]
- 45. Verhoef TI, Redekop WK, Bouvy ML, et al. Beliefs about medicines in Dutch acenocoumarol and phenprocoumon users. Br J Clin Pharmacol. 2014;78:422–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Platt AB, Localio AR, Brensinger CM, et al. Risk factors for nonadherence to warfarin: results from the IN‐RANGE study. Pharmacoepidemiol Drug Saf. 2008;17:853–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Gomes T, Mamdani MM, Holbrook AM, Paterson JM, Juurlink DN. Persistence with therapy among patients treated with warfarin for atrial fibrillation. Arch Intern Med. 2012;172:1687–9. [DOI] [PubMed] [Google Scholar]
- 48. Kaptein AA, van Korlaar IM, Cameron LD, Vossen CY, van der Meer FJ, Rosendaal FR. Using the common‐sense model to predict risk perception and disease‐related worry in individuals at increased risk for venous thrombosis. Health Psychol. 2007;26:807–12. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


