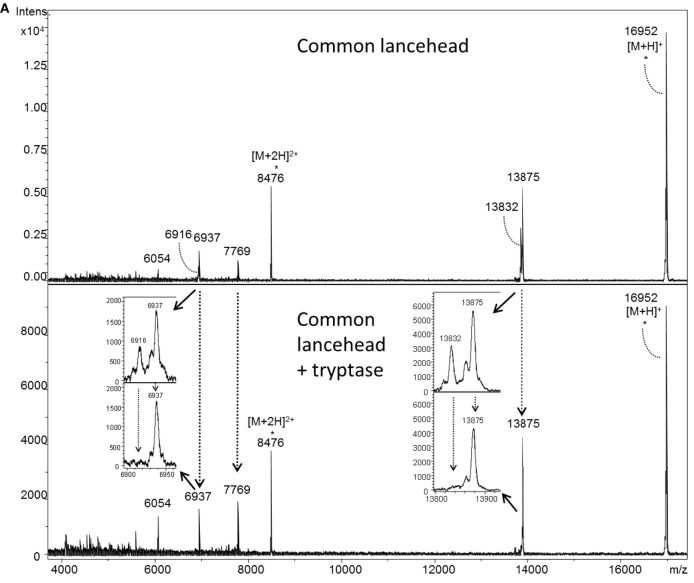
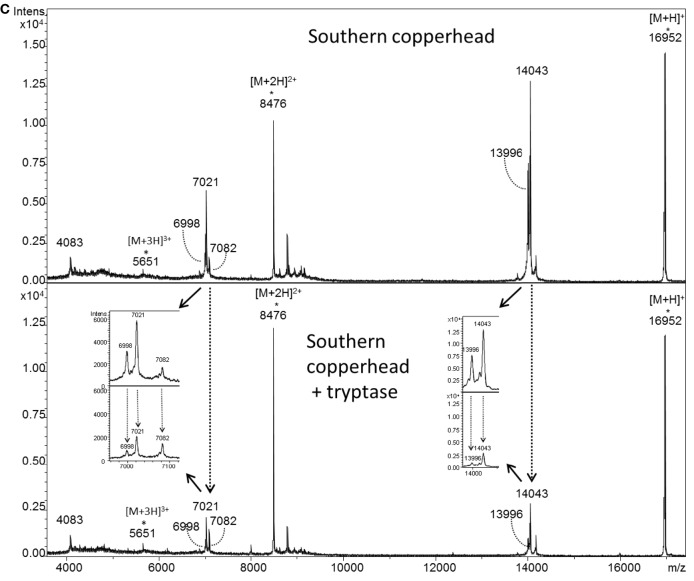
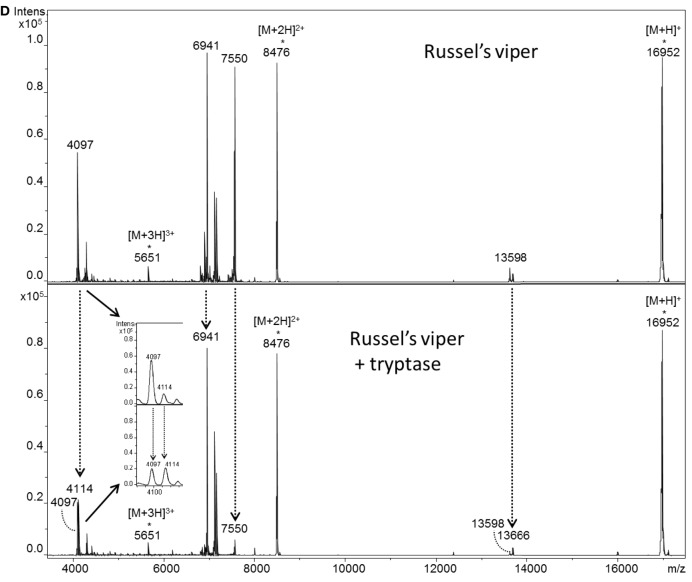
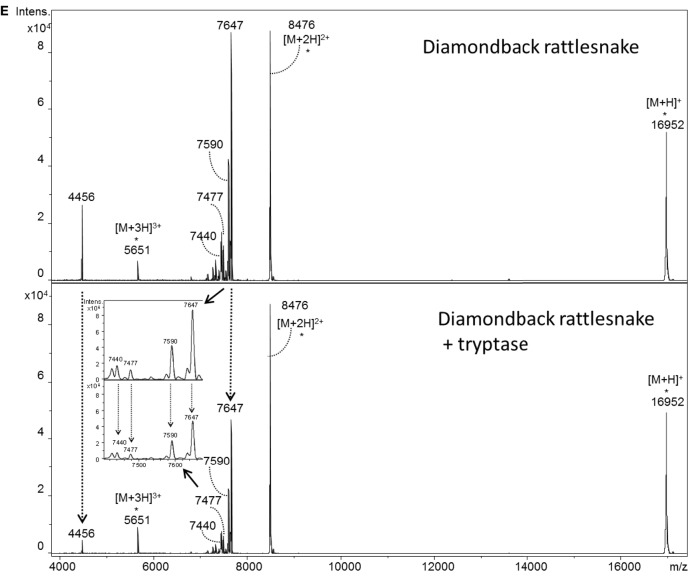
Figure 5.
MALDI-TOF-MS analyses of whole venoms treated with purified human tryptase shows degradation of venom proteins. Venoms from five snake species [(A) common lancehead, (B) saw-scaled viper, (C) southern copperhead, (D) russel’s viper, (E), western diamondback rattlesnake] were treated with tryptase or vehicle control. Spectra were acquired within the mass range of m/z 3,500 to m/z 18,000. Samples contain equal amounts (0.5 µg) myoglobin as an internal reference (m/z 16,952 [M + H]+, m/z 8,476 [M + 2H]2+, m/z 5,651 [M + 3H]3+). Venom-derived signals (dashed arrows) distributed over the whole mass range were selected and normalized to the intensity of the doubly charged myoglobin signal (Figure 4B). The reduction in relative signal intensities of several peaks in tryptase-treated versus untreated venom indicates tryptase-mediated protein degradation.