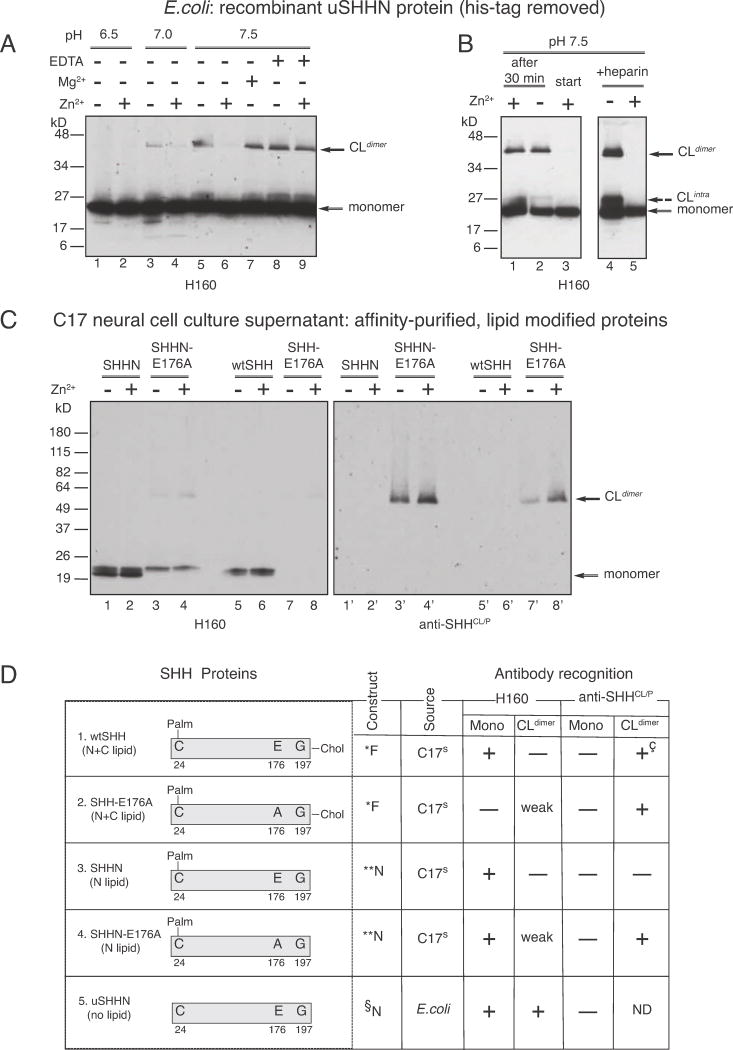
Figure 3. SHH-E176 controls Zn2+-dependent cross-linking and recognition by conformation-specific antibodies.
Factors affecting the formation of SHH non-reducible cross-linked dimers (CLdimer) A. uSHHN (lanes 1–9), lanes 1–2, pH 6.5, lanes 3–4, pH 7.0, lanes 5–9, pH 7.5, lanes 2, 4, 6, 9, 1 mM Zn2+, lane 7, 1 mM Mg2+, lanes 8 and 9, 1 mM EDTA. Western analysis was performed using anti-SHH antibody H160. B. uSHHN (lanes 1–5), lane 1, Zn2+ addition 30 minutes after cross-linking reaction starts, lane 2, minus Zn2+, lane 3, Zn2+ addition from the beginning of the cross-linking reaction, lanes 4–5, 100ng heparin, lane 5, 1 mM Zn2+. Western analysis was performed using anti-SHH antibody H160. Solid arrows indicate CLdimer and monomer, and dashed arrow indicates CLintra. C. SHHN proteins affinity purified from secreted cell culture supernatants, affinity purified on a 5E1 column (5E1, monoclonal anti-SHH, Developmental Hybridoma Studies Bank), and assayed for cross-linking. SHHN (lanes 1–2, 1′–2′), SHHN-E176A (lanes 3–4, 3′–4′), wtSHH (lanes 5–6, 5′–6′), and SHH-E176A (lanes 7–8, 7′–8′) were incubated in the presence (lanes 2, 4, 6, 8) or absence (lanes 1, 3, 5, 7) of 1mM Zn2+. Western analysis was performed using α-SHHCL/P (lanes 1′–8′), stripped and reprobed with H160 (lanes 1–8). Arrows indicate CLdimer and monomer. D. Description of recombinant proteins used in A–D: *Full-length construct, produces N-and C-lipid modified SHH proteins. SHH proteins are affinity purified from culture supernatants of transfected C17 neural cells, as previously described for wtSHH (Feng et al., 2004), **N-terminal construct produces N-lipid containing, C-lipid lacking SHH proteins. SHH proteins are affinity purified from supernatants of transfected C17 neural cells, previously described for (Feng et al., 2004), §N-terminal construct lacks both N-and C-lipid modifications. uSHHN is produced in E.coli, Ni-Agarose affinity purified, histidine tag cleaved, as previously described in (Williams et al., 1999). + (antibody recognizes), − (antibody does not recognize), weak (antibody weakly recognizes), ND (not determined), +ç (recognizes wtSHH crosslinked form after storage at −80°C for 6 months). Note: Recombinant proteins (1–5) do not contain tags at the N-or C-termini. Proteins 1–4 were purified by affinity chromatography on a 5E1 anti-SHH-antibody column, while the 6Xhis-tag on uSHHN (protein 5) was removed prior to crosslinking assays.