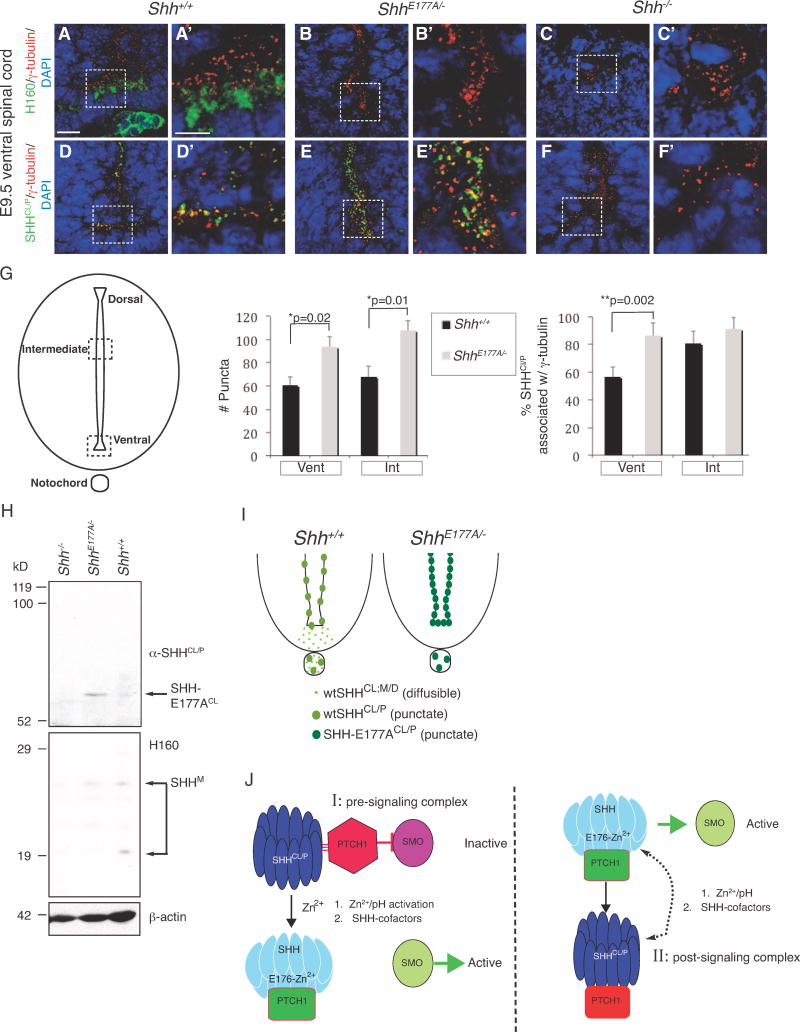
A–F′. Confocal analysis of immunofluorescence staining using H160 (A–C′, green) and α-SHH
CL/P (D–F′, green), on E9.5
Shh+/+ (A, A′, D, D′),
ShhE177A/− (B, B′, E, E′), and
Shh−/− (C, C′, F, F′) lumbar spinal cord sections. Scale bars 10μm (A–F), 5μm (A′–F′).
G. Schematic of the spinal cord highlighting the regions where SHH
Cl/P puncta are counted. Bar graph on the left shows the total number of puncta per 2000μm
2 area; bar graph on the right shows the %SHH
CL/P associated with γ-tubulin. Counting is performed in ventral (Vent) and intermediate (Int) regions of the
Shh+/+ (black bars) and
ShhE177A/− (gray bars) E9.5 lumbar spinal cord. In
Shh+/+, the dorsal boundary of the intermediate region is 200 μm from the dorsal edge of the spinal cord; the ventral boundary of the intermediate region is 320 μm from the ventral edge of the spinal cord. A corresponding region is used for
ShhE177A/− embryos. Data represented as mean ± SD, Student’s
t-test, total number of puncta:
*p=0.02.(ventral region) and
*p= 0.01, (intermediate region), %SHH
CL/P associated with γ-tubulin
*p=0.002 (ventral region). n=3 embryos for each region.
H. Western analysis of membrane extracts isolated from anterior region of
Shh+/+, ShhE177A/−, and
Shh−/− E10.5 embryos. The top of the blot was probed with α-SHH
CL/P and bottom was probed with H160. The lower part of the blot was reprobed with anti-β-actin for a loading control. A long gel (14 cm) was run for better protein separation. α-SHH
Cl/P identifies SHH-E177A
CL in
ShhE177A/−, indicated by the arrow. H160 identifies SHH monomers migrating as multiple bands between 19–29 kD (SHH
M). There is a shift from the 20kD monomer to the cross-linked form in
Shh+/+ compared to
ShhE177A/−. Shh−/− extracts are loaded as a negative control.
I. Ventral spinal cord schematics show that
ShhE177A/− mutants lack diffuse Shh staining (light green) in floorplate, with increased puncta (dark green) expression in the spinal cord ventricle.
J. Models proposing possible roles of SHH-E177, Zn
2+ and SHH/
CL/P in SHH signaling.
I. Pre-signaling E176/E177-Zn
2+ activation model of SHH signaling at cilia BBs.
II.
Post-signaling model of SHH
CL/P formation at cilia BBs. While the pre-signaling model is preferred based on the findings listed below, it is also possible that SHHCL/P can function in both pre and post-signaling. The model is based on the following findings:
E176A/E177A modulates Zn2+-mediated conformational change, detected by increased formation of cross-linked dimers.
E176A/E177A is active at ectopic, but not endogenous sites in vivo.
In ShhE177A/− mutant spinal cord, increased accumulation of SHH-E177A occurs near cilia BBs.
SHHCL/P is still present in the ventral spinal cord even in the absence of the SHH receptor PTC1, supporting a pre-signaling role of SHHCL/P.