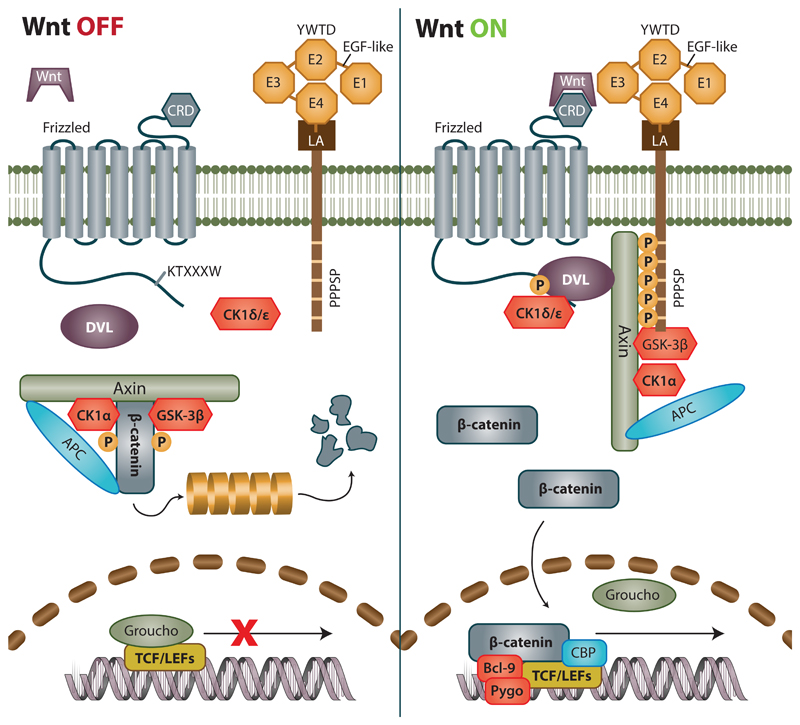
Figure 1. Current view of Wnt/β-catenin signaling in OFF and ON state.
During OFF state destruction complex consisting of Axin, APC and GSK-3β phosphorylates β-catenin and marks it for subsequent degradation via ubiquitin proteasome pathway. At the same time, transcription factors from the TCF/LEF family remain bound to repressors such as Groucho, blocking the transcription of Wnt target genes. Cascade is activated after binding of Wnt ligand to Frizzled (Fzd) receptor. Subsequently, both DVL and Lrp6 associate to Fzd. Intracellular residues of Lrp6 are phosphorylated and become a site of attachment for scaffold protein Axin, which can no longer serve as assembly site for destruction complex, which is thus desintegrated. It should be noted that phosphorylated Lrp6, DVL Axin together with other proteins form a structures dubbed signalosomes that ‘attract’ each other and amplify the Wnt signal. As a result, β-catenin is no longer degraded, and accumulates in the cytoplasm. After reaching a certain threshold, it is translocated to nucleus where it binds to TCF/LEF family of transcription factors, replaces resident repressors thereby co-activating transcription of its target genes.