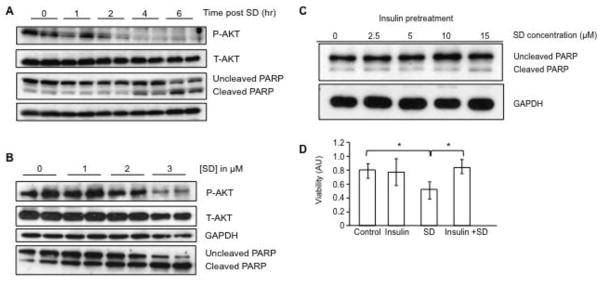
Figure 3. SD mediate cytotoxicity in neuroblastoma cells by inhibiting AKT signaling.
(A) Kelly cells were grown to 70% confluence in the preferred medium plus 10% FCS, switched to serum-free medium, treated with 10 μM SD and incubated for an additional 0, 1, 2, 4 or 6 h. Cells were harvested at the designated time point, and whole cell extracts were analyzed by immunoblotting for total AKT (T-AKT) and phosphorylated AKT (P-AKT) and cleaved and uncleaved PARP. (B) Kelly cells were grown as described in (A) and after incubation in serum-free medium for 8 h were then treated with 0–3 μM SD for 20 h. Cells were harvested and whole cell extracts were analyzed by immunoblotting for T-AKT, P-AKT and cleaved and uncleaved PARP. (C) Kelly cells were serum starved for 12 h, pre-treated with insulin (2 μg/ml) for 1 h, followed by treatment with SD (0–15 μM). After 6 h of incubation, cells were harvested, and cleaved and uncleaved PARP were measured by immunoblotting of whole cell extracts. GAPDH was used as loading control. (D) Kelly cells received either no pre-treatment (control), insulin (2 μg/ml) pre-treatment for 1 h to activate AKT signaling, 10 μM SD without pre-treatment, or insulin pre-treatment followed by SD treatment. Cells were incubated for an additional 6 h, and cell viability was determined by the MTS assay. * p < 0.05. These experiments were repeated at least three times with similar results.