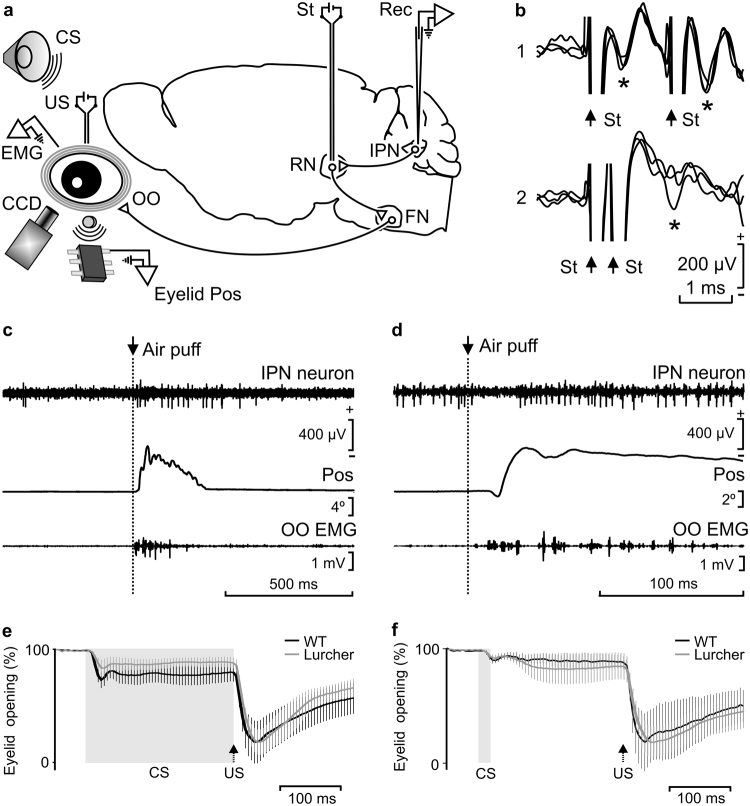
Figure 1.
Experimental design and identification of recorded IPN neurons. (a) Diagrammatic representation of the experimental design. Mice were chronically implanted with stimulating electrodes on the left supraorbital nerve for US presentations and with EMG recording electrodes in the left orbicularis oculi (OO) muscle. A loudspeaker was used for the presentation of a tone as CS. Eyelid position (Eyelid Pos) was determined as the voltage difference between a Hall-effect sensor located on the head-holding system and a magnetic piece fixed to the lower eyelid. In addition, eyelid opening was detected with a fast CCD camera. Ipsilateral IPN neurons were recorded (Rec) with glass micropipettes and identified by their antidromic stimulation (St.) from the contralateral red nucleus (RN). FN, facial nucleus. (b) Overlapped (n = 3) traces of the antidromic activation (*) of a representative IPN neuron from the RN (St.) at threshold-straddling intensities and at different (1, 1.6 ms; and 2, 0.4 ms) interstimulus intervals. Note in 2 that the antidromic activation is partially prevented. Arrows indicate stimulus artifacts. (c,d) Representative examples of the firing rate of type A (c) and B (d) IPN neurons following the presentation of an air puff aimed at the ipsilateral cornea. Eyelid position (Pos) and OO EMG are also illustrated. (e,f) Typical CRs of trained WT and Lurcher animals collected during delay (e) and trace (f) conditioning paradigms. Values are mean ± SEM of eyelid closing percentages (n = 6 animals) obtained by a MATLAB-based analysis of the photographs taken with a fast CCD camera. Gray bands and black arrows represent CS duration and US presentation respectively. Note the different patterns of eyelid closing for delay and trace conditioning paradigms, but not between WT and Lurcher mice.