Fig. 6.
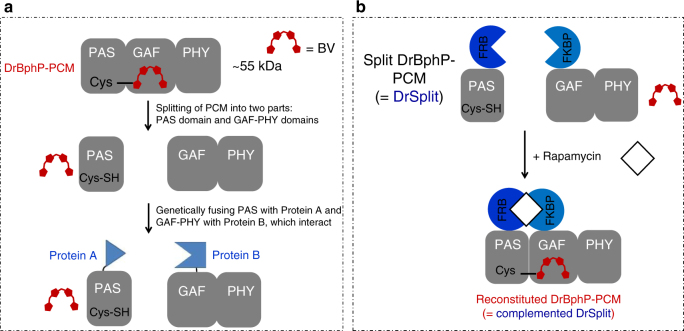
Development of the bimolecular photoacoustic complementation (BiPC) reporter DrSplit. a DrBphP-PCM consists of three domains, PAS, GAF, and PHY. The biliverdin (BV) chromophore is covalently bound with conservative cysteine from the PAS domain and secured to a chromophore-binding pocket in the GAF domain. DrBphP-PCM was genetically split into two parts, the PAS domain and GAF-PHY domain, together named DrSplit. In this case, BV does not bind with any part of DrSplit. Genetically fusing one protein of interest (protein A) to one part of DrSplit and another protein of interest (protein B) to another part of DrSplit makes possible the monitoring of protein–protein interactions (PPIs) between protein A and protein B. b We used a model rapamycin-induced PPI between the FRB and FKBP proteins for evaluation of DrSplit. FRB was fused to the PAS domain and FKBP was fused to the GAF-PHY domains. Upon addition of rapamycin to the DrSplit, DrBphP-PCM was re-functionalized