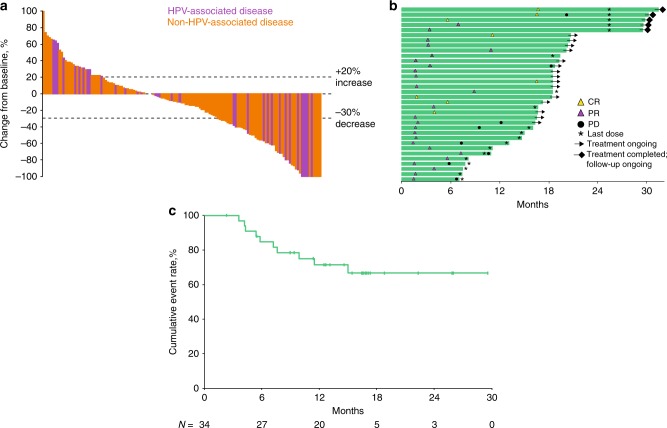
Fig. 2.
Tumour response to pembrolizumab according to RECIST v1.1 by central imaging vendor review. a Best percentage change from baseline in target lesions (n = 139). Includes patients who had measurable disease at baseline and at least one post baseline scan. b Treatment exposure and duration of response in patients achieving partial responses or complete responses (n = 34). c Kaplan–Meier estimate of the duration of response in patients achieving partial responses or complete responses. RECIST Response Evaluation Criteria in Solid Tumors