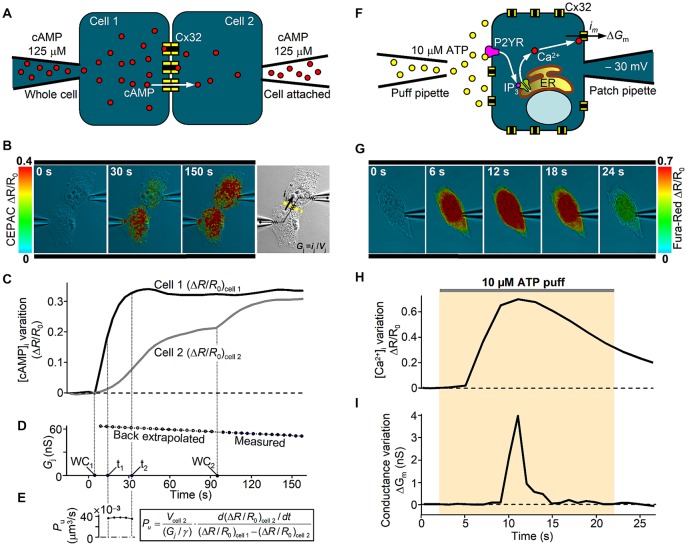
Figure 2.
In vitro analysis of human Cx32 GJ and hemichannel functionality. (A) Scheme of dual patch clamp experiment to derive the unitary GJ permeability to cAMP in an isolated pair of Cx32-WT transfected HeLa cells, as in Carrer et al. (2018). At time zero, cAMP is injected in cell 1 under whole-cell recording conditions (WC1) and its intercellular transfer is monitored by FRET variation (ΔR/R0) of CEPAC sensor. (B) Three representative frames illustrate successive stages of an experiment. Around 90 s after WC1, the whole-cell configuration is achieved also in cell 2 (WC2), delivering the same concentration of cAMP and deriving the junctional conductance Gj from the current ij elicited by a 10 mV voltage difference (Vj) between the two pipettes, as illustrated in the bright field image. (C,D) Time course of the cell-averaged FRET signal and the junctional conductance Gj from the experiment in (B). (E) A confocal z-stack was performed at the end of the experiment to derive cell 2 volume (Vcell 2), which is required to compute the single channel permeability Pu to cAMP, as described in Hernandez et al. (2007), where γ is the single channel conductance. (F) Scheme of patch clamp experiment to study the hemichannel gating by [Ca2+]i in a single Cx32-WT transfected HeLa cell. An IP3-dependent [Ca2+]i transient is stimulated by an extracellular puff containing 10 μM ATP. Cx32 hemichannel opening and closure were monitored in terms of membrane conductance variation (ΔGm) computed by the periodic application (at 1 Hz) of a +10 μV voltage step lasting 100 ms. Contribution to ΔGm by other Ca2+-activated channels was kept negligible by specific blockers contained in the extracellular solution. (G) Representative frame sequence of an experiment using Fura-Red dye. (H,I) Time course from the experiment in (G) of the cell-averaged Fura-Red ΔR/R0 and the membrane conductance variation ΔGm due to opening and closure of Cx32-WT hemichannels. For further details, see Carrer et al. (2018).