FIG 4.
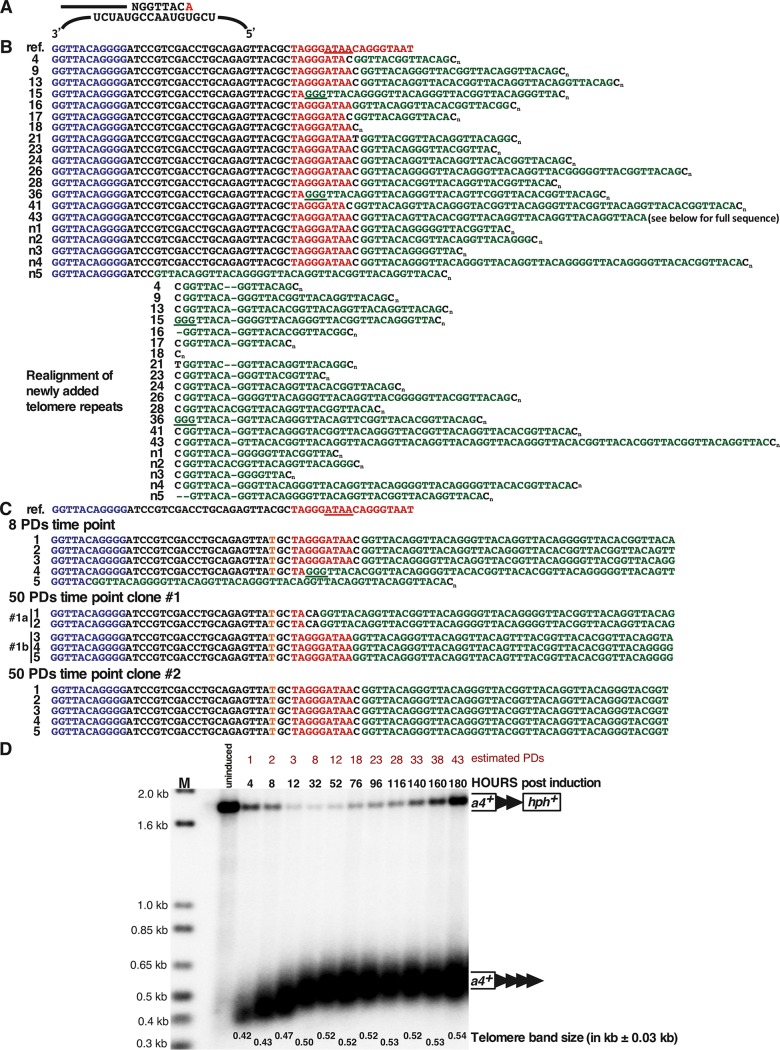
Analysis of the newly added telomere repeats at the 48-bp prototelomere. (A) Sequences added by S. pombe telomerase in the first round of synthesis. The top line represents a DNA strand further elongated by telomerase, where the added GGTTACA sequence hybridizes to the telomerase RNA template (bottom strand) (53). The red A at the 3′ end may or may not be added. (B) Sequences of newly added telomere repeats from cells at the ∼1-PD time point (4.2 h postinduction). The top row (ref.) shows the reference sequence of the 48-bp prototelomere. The sequences in blue represent part of the 48 bp of telomere repeats, black is the polylinker sequence, red is the I-SceI site, with the underlined red bases representing the overhang after I-SceI cleavage, and green is the newly added telomere repeats. Cn indicates the oligo(dC) added during the telomere PCR amplification (87). The 20 rows below are sequences from 20 individual clones collected at the ∼1-PD time point. The realigned sequences separate the first newly added telomere repeat, NGGTTAC(A), of all the telomeres from the 3′ sequences. The underlined GGG sequences may have been part of the original I-SceI site or added by telomerase. Interestingly, all telomere repeat addition was to the I-SceI site or polylinker sequences, similar to telomerase-mediated repeat addition in S. cerevisiae (54, 55, 94) and mammalian cells (38, 56). (C) Telomere sequences cloned from the ∼8-PD time point or different clones from the ∼50-PD time point. These fully elongated telomeres still retain the polylinker and I-SceI site in all but one case, indicating that this conformation forms a stable telomere. A C-to-T point mutation in the polylinker sequence in the clones is highlighted in orange. The ∼50-PD clone 1 appears to be a mixture of two clones, as two sequences (1a and 1b) were rescued from the culture. Only the telomere repeat sequences closest to the addition site are shown. (D) Telomere repeat tracts are fully elongated by ∼8 PDs after prototelomere cleavage. After induction of I-SceI, cells were grown for multiple PDs in liquid culture with 9 μM ahTET by serial dilution, and samples from different time points were processed for Southern blotting using ura4+ as a probe, as for Fig. 3A. The modal terminal restriction fragment (TRF) sizes of the newly formed telomere (ura4+ telomere repeat band) after induction was measured at the most intense hybridizing point in the band. Band sizes on these blots vary by approximately ±0.03 kb. Molecular size standards are shown (lane M). The data reveal that cells with an uncleaved prototelomere had a growth advantage over cells with the new telomere, so that the cells with the uncleaved prototelomere increased in proportion during continuous growth. The uncleaved prototelomeres most likely resulted from cassettes that were cut and healed by a DNA repair event that eliminated the I-SceI site.