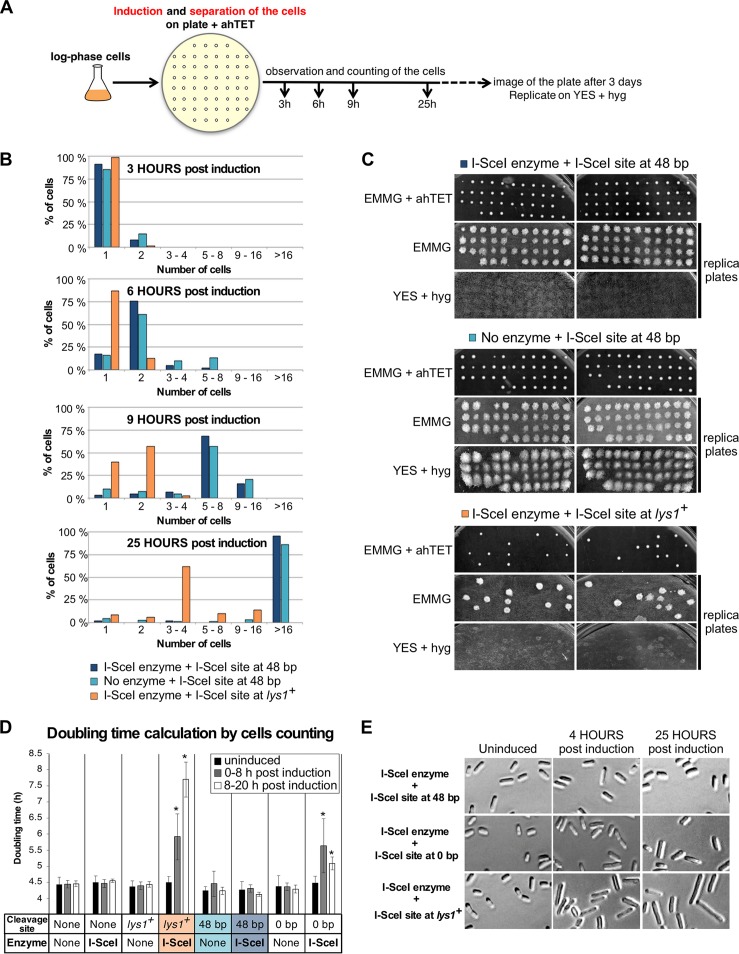
FIG 7.
Cleaving the 48-bp prototelomere does not induce a cell cycle pause. (A) Experimental design. Telomere formation was induced on synthetic nonselective plates supplemented with 9 μM ahTET. Single cells (40 to 44) were micromanipulated onto a grid, and cell numbers were observed after incubation for 3, 6, 9, and 25 h at 32°C. The plates were incubated for an additional 3 days, scanned, and then replicated on nonselective plates and on YES-plus-hygromycin plates, which were then grown for 3 days at 32°C and scanned. (B) Numbers of cells of different strains at 3 to 25 h postinduction. Cells bearing the I-SceI site at 48-bp prototelomeres with or without the I-SceI expression cassette or cells with an I-SceI site at lys1+ and the I-SceI expression cassette were assayed. (C) Cells from panel B were grown for 3 days at 32°C, replica plated to the indicated media, and grown for 3 more days at 32°C. Images of the original plate (EMMG plus ahTET) and the two replica plates are shown for each strain. (D) Doubling times of the strains with the 48-bp or 0-bp prototelomere or the lys1+ I-SceI site with or without the I-SceI expression cassette. Doubling times from three independently induced cultures were calculated by counting cells using a hemocytometer at the indicated time points. More than 1,000 cells were counted for each genetic construction. The doubling times of different strains were compared by t test to that of the wild-type strain lacking an I-SceI site and the I-SceI expression cassette. Significant differences (P < 0.05) are marked by asterisks. (E) Representative images of cells bearing the I-SceI site at the 48-bp or 0-bp prototelomere or at lys1+. Growing cells prior to induction (uninduced) or 4 or 25 h postinduction were photographed at the same magnification to compare cell sizes after I-SceI cuts at the different loci. The strain with a lys1+ I-SceI site shows the elongation phenotype characteristic of G2/M arrest due to DNA damage.