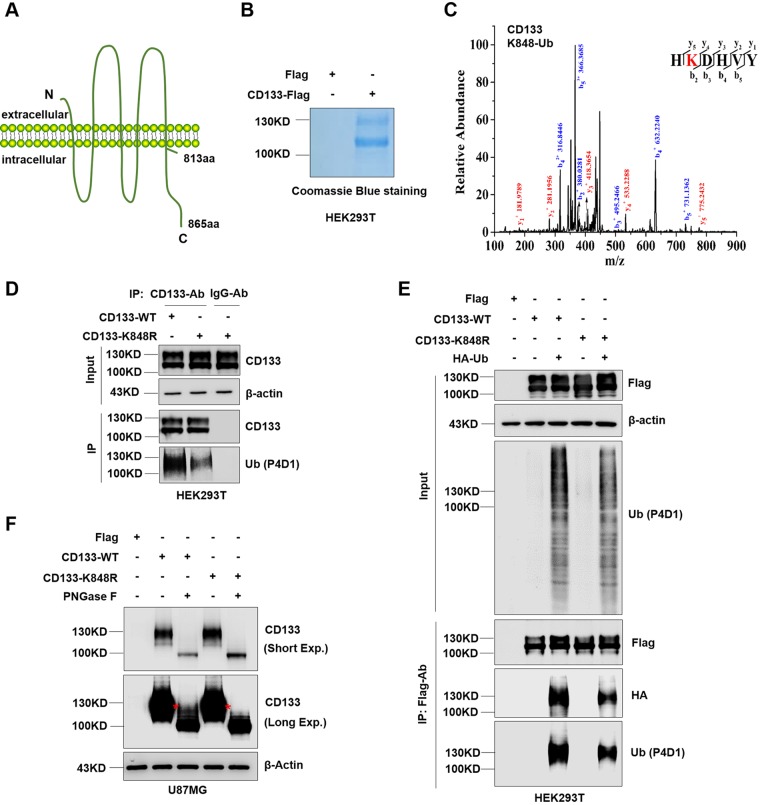
FIG 3.
Complex glycosylated CD133 is ubiquitinated at Lys848. (A) Proposed structural model of CD133. (B) Purity of CD133 protein from HEK293T cells, determined by Coomassie blue staining. (C) MS analysis showed complex glycosylated CD133 (≈130 kDa) to be ubiquitinated at Lys848. The multiple lines are the fragment ions that confirm K848 as the ubiquitination site. (D) The K848R mutant or wild-type (WT) plasmid was expressed in HEK293T cells, and immunoprecipitation was performed using a CD133 antibody. Normal mouse IgG antibody was used as a negative control. CD133 ubiquitination was detected by Western blotting; β-actin was blotted as a loading control. (E) Flag-tagged CD133-WT or CD133-K848R was coexpressed with HA-Ub in HEK293T cells, followed by IP-Western blot analysis. (F) U87MG cells were used to stably express Flag, CD133-WT, or CD133-K848R. Cell lysates were treated with PNGase F for deglycosylation and then subjected to Western blotting. β-Actin was blotted as a loading control. All results were collected from three independent experiments. aa, amino acids; MS, mass spectrometry; IP, immunoprecipitation; Exp., exposure.