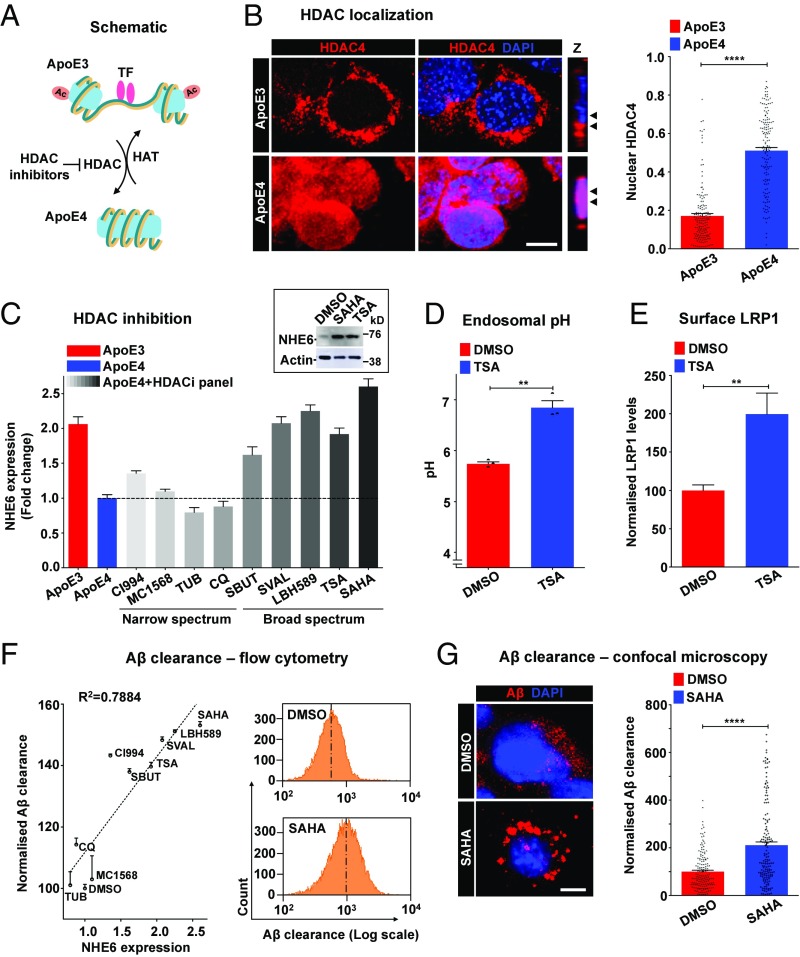
Fig. 5.
HDAC inhibitors rescue NHE6-mediated Aβ clearance deficits. (A) Schematic showing HDAC activation in ApoE4, compared with increased histone acetylase (HAT) activity and transcription factor (TF) binding in ApoE3. HDAC inhibitors could potentially exert therapeutic effects by reducing ApoE4-induced nuclear translocation of HDACs. (B) Representative micrographs (Left) and quantification using the Pearson correlation coefficient (Right) of colocalization of HDAC4 (red) with nuclear DAPI (blue) in ApoE3 and ApoE4 astrocytes. Colocalization is evident in the merge and orthogonal slices (Z) as magenta puncta. Note the prominent overlap between HDAC4 and DAPI, consistent with increased nuclear translocation, in ApoE4 astrocytes (Pearson’s correlation coefficient for ApoE3: 0.17 ± 0.01 vs. ApoE4: 0.51 ± 0.02; ****P = 4.6 × 10−44, Student’s t test; n = 150 per condition). (C) Quantitative PCR analysis to determine the efficacy of HDAC inhibitors to augment the expression of NHE6 in ApoE4 astrocytes following 12 h of treatment. Broad-spectrum drugs, including sodium butyrate, sodium valproate, LBH589, TSA, and SAHA, restored NHE6 expression in ApoE4 astrocytes to levels comparable to ApoE3 astrocytes. (Inset) Robust increase of NHE6 protein with HDAC inhibition by SAHA and TSA treatment. The complete Western blot is shown in SI Appendix, Fig. S8B. (D) HDAC inhibition by TSA treatment (5 μM for 12 h) corrected hyperacidic endosomal pH in ApoE4 astrocytes, relative to DMSO treatment (**P = 0.0014, Student’s t test; n = 3). (E) HDAC inhibition by TSA treatment significantly increased surface levels of LRP1, as determined by surface labeling at 4 °C and flow cytometry, in ApoE4 astrocytes (**P = 0.0037, Student’s t test; n = 3). The fluorescence-activated cell sorting (FACS) histogram is shown in SI Appendix, Fig. S8G. (F, Left) Quantitation of Aβ clearance by FACS analysis (10,000 cells in biological triplicates) to determine the efficacy of HDAC inhibitors to rescue Aβ clearance deficits in ApoE4 astrocytes. Note the prominent linear relationship between Aβ clearance and the fold change in NHE6 expression (R2 = 0.7884, P < 0.0001) elicited by DMSO and nine HDAC inhibitors. CQ, clioquinol; SBUT, sodium butyrate; SVAL, sodium valproate; TUB, tubacin. (Right) Representative FACS histograms demonstrating the increase in Aβ internalization by ApoE4 with SAHA treatment. (G) Representative micrographs (Left) and quantification (Right) demonstrating internalized Aβ following SAHA treatment. Note the prominent, vesicular Aβ staining in SAHA-treated ApoE4 cells (****P = 9.9 × 10−13, Student’s t test; n = 160 per condition) (SI Appendix, Fig. S8). (Scale bars, 10 μm.)