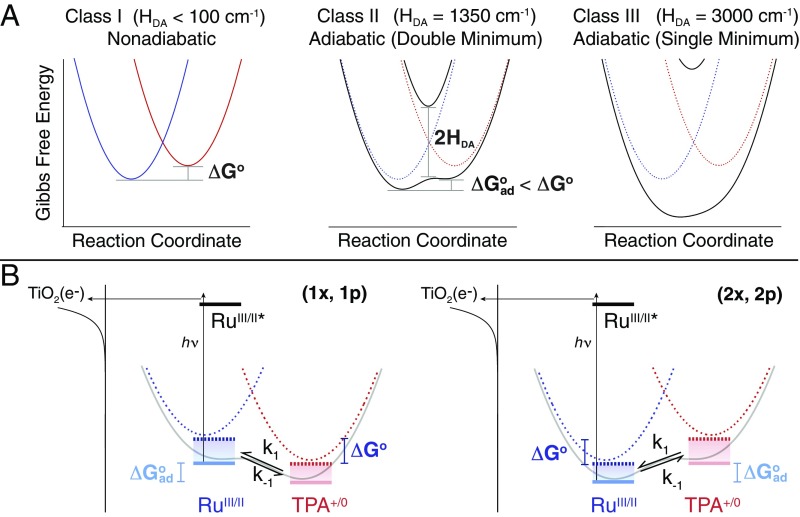
Fig. 2.
The Gibbs free-energy surfaces and the kinetic approach. (A) GESs that represent a redox equilibrium between A-B-D (blue) and A−-B-D+ (red) as the electronic coupling matrix element (HDA) is increased from 0 (nonadiabatic) to over 3,000 cm−1 (adiabatic). Emphasis is placed herein on the reduction in the Gibbs free-energy change, |ΔGo| > |ΔGoad| that accompanies the transition from nonadiabatic to adiabatic electron transfer in the double-minimum regime. (B) The approach consists of a RuII-B-TPA compound anchored to the surface of mesoporous thin films of TiO2 (the secondary acceptor). Light absorption induces excited-state electron injection from the RuII unit into the TiO2 to form TiO2(e−)|-RuIII-B-TPA. Within the timeframe of charge recombination, the dynamic equilibrium RuIII-B-TPA RuII-B-TPA+ was quantified through a kinetic model that afforded the forward, k1, and reverse, k−1, electron-transfer rate constants.