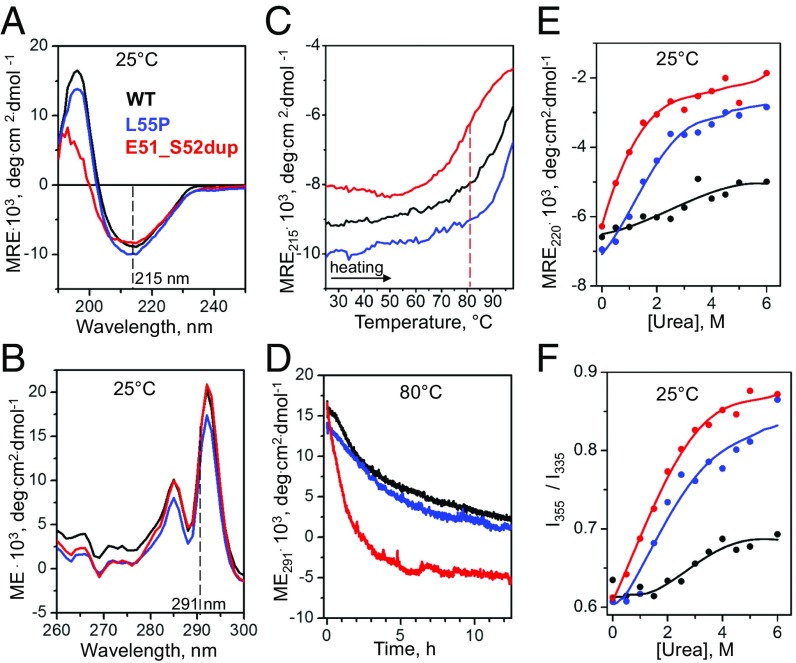
Fig. 2.
Effects of the duplication mutation on the TTR secondary and tertiary structure and stability. Recombinant proteins were analyzed by CD and fluorescence spectroscopy as described in Materials and Methods. WT (black) and Leu55Pro (blue) were used as controls for Glu51_Ser52dup (red). (A) Far-UV CD spectra (0.2 mg/mL protein in standard buffer at 25 °C) characterize protein secondary structure. (B) Near-UV CD spectra (0.2 mg/mL protein in standard buffer at 25 °C) show a double peak characteristic of Trp79 buried in the core of the native TTR monomer (2). (C) Melting data recorded by CD at 215 nm to monitor β-sheet unfolding during heating from 25 to 98 °C at a rate of 6 °C⋅h−1. Sample conditions are as in A. The vertical dashed line indicates the apparent melting temperature for Glu51_Ser52dup, Tm, app = 81 °C, determined from the first-derivative maximum in the melting data. (D) Time course of protein unfolding in a temperature jump to 80 °C. At t = 0, the sample temperature was rapidly increased from 25 to 80 °C, and changes in the Trp solvent exposure upon protein unfolding were monitored by CD at 291 nm. Sample conditions are as in B. (E) Equilibrium denaturation by urea monitored by CD at 220 nm for secondary structure unfolding after incubation in urea for 96 h. Protein concentrations are 0.2 mg/mL. (F) Equilibrium denaturation by urea tracked by the ratio of fluorescence intensity at 355 nm and 335 nm, I355/I335, to monitor solvent exposure of Trp. Sample conditions are 0.2 mg/mL protein. deg, degrees; ME, molar ellipticity; MRE, molar residue ellipticity.