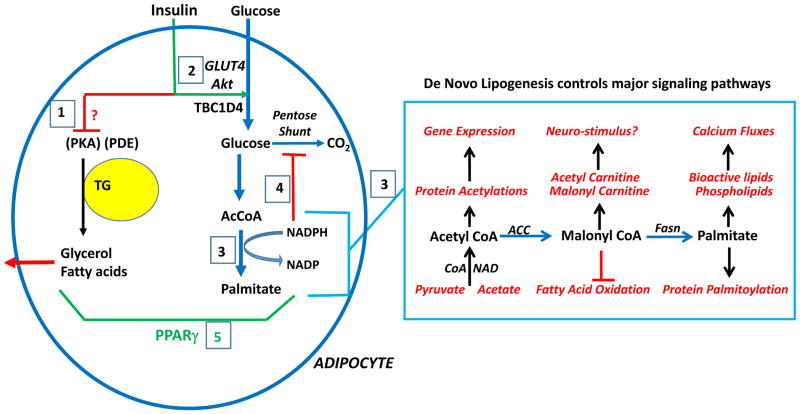
Figure 5. Adipocyte pathways downstream of insulin signaling to Akt disrupted by HFD/obesity that may contribute to systemic insulin resistance in mice and humans.
Contained within the circle at left are pathways of glucose and lipid metabolism that become impaired in their response to insulin in obesity. Insulin inhibits hydrolysis of triglyceride (TG) to glycerol and fatty acids (boxed 1) by a mechanism independent of phosphodiesterase (PDE) phosphorylation by Akt that remains a major unknown and key challenge in the field. Also unknown is how disruption of this anti-lipolytic effect of insulin in obesity is impaired. Insulin enhances glucose uptake into adipocytes through Akt-dependent translocation of Glut4 glucose transporters to the plasma membrane (boxed 2). Adipocyte Glut4 expression is decreased in obesity, and this is mimicked by chronic insulin stimulation in vitro, suggesting that hyperinsulinemia may contribute to this effect in vivo. Adipocyte de novo lipogenesis (DNL) (boxed 3) is markedly inhibited in HFD/obesity by mechanisms that are not defined. According to this model, DNL inhibition through feedback inhibition by NADPH decreases glucose flux through the pentose shunt (boxed 4), contributing to increased free glucose and decreased glucose uptake. PPARγ (boxed 5) is a master regulator of adipocyte genes, thus attenuation of its activity in obesity has the potential to cause adipocyte dysfunction and insulin resistance. Large rectangle at right illustrates the many ways that intermediates of DNL may act as signaling molecules to regulate gene expression and many other cellular functions, for example through histone and other protein acetylations, allosteric modulation, protein palmitoylation and generation of bio-active lipids.