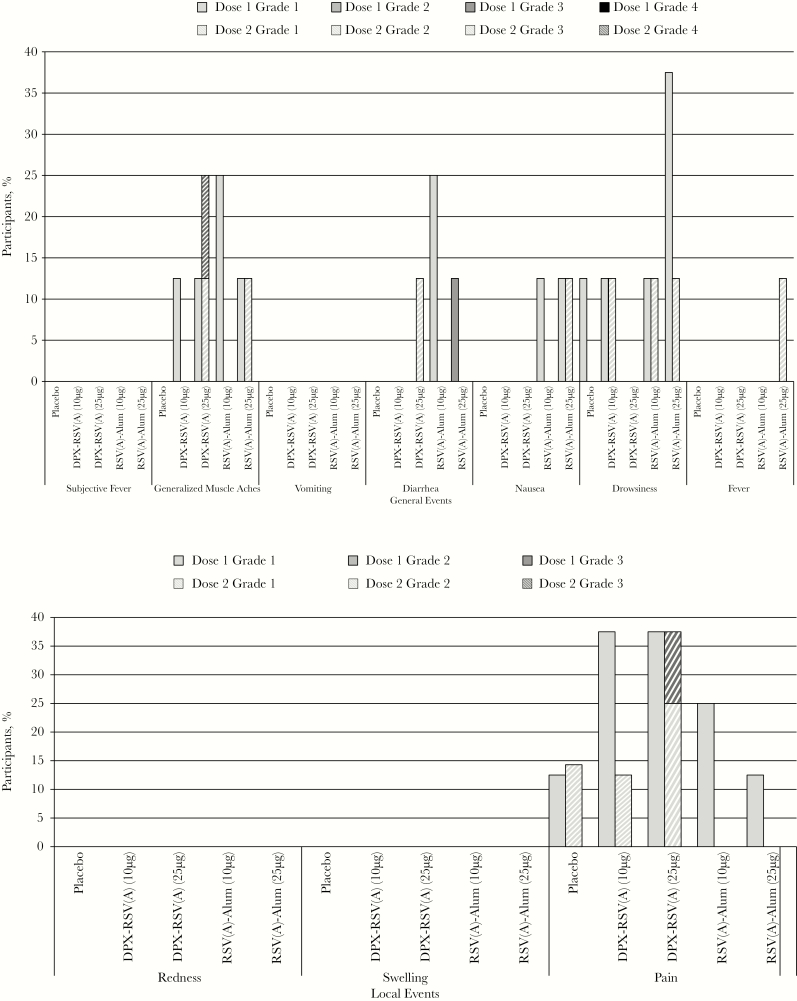
Figure 2.
Percentage of participants with solicited injection site and general adverse events on days 0–6 after vaccination (doses 1 and 2 at days 0 and 56). Grade 3 (severe) pain was defined as pain that is significant at rest and prevents normal everyday activities. Redness and swelling were considered present if the greatest surface diameter of each was >100 mm. Grade 3 (severe) systemic adverse events were defined as those that prevent normal everyday activities. aParticipants in the RSV(A)-Alum group received placebo on day 56, rather than RSV(A)-Alum. See “Methods” for a description of vaccine formulations. DPX, DepoVax; RSV(A), respiratory syncytial virus subgroup A.