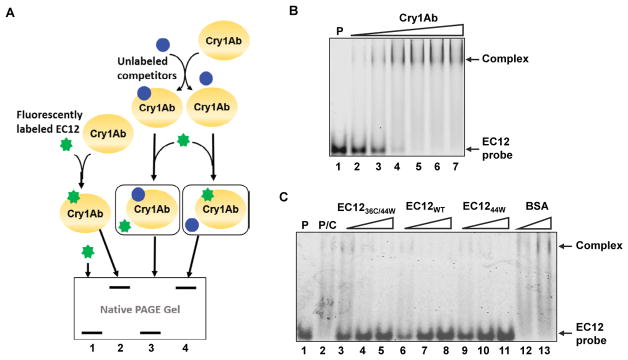
Figure 4.
Detection of competitive binding with fluorescently labeled EC12.
(A) Schematic of competitive binding reactions between EC12 and Cry1Ab. Labeled EC1236C/44W (EC12 probe or probe) is depicted as a green “star” and, when not bound to Cry1Ab, appears as fluorescent bands at the bottom of a native gel (lanes 1 and 3). On the other hand, binding of the probe to Cry1Ab results in the formation of a protein complex shown as fluorescent bands at the top of a native gel (lanes 2 and 4). It is feasible that potential competitors (blue circles) could bind to Cry1Ab prior to the addition of the EC12 probe. As seen in Fig. 4A, two alternatives are possible. One alternative is that a competitor occupies the same binding site for the probe on the Cry1Ab toxin, preventing its binding to Cry1Ab (lane 3). The other alternative is that a protein may not be able to bind to Cry1Ab, thus allowing binding of the probe to the toxin (lane 4).
(B) A representative fluorescent image demonstrating the binding of EC12 probe to Cry1Ab. Lane 1 (P): 2 μM EC12 probe; Lanes 2 to 7: 2 μM EC12 probe incubated with 1, 2, 3, 4, 5 and 6 μM Cry1Ab, respectively. (C) Competition binding experiments to determine the binding specificity of EC12 to Cry1Ab toxin. A representative fluorescent image of indicated protein samples on a native gel is shown. In these reactions, unlabeled EC12 molecules and bovine serum albumin (BSA) with various molar ratios to the EC12 probe was incubated with Cry1Ab (500 nM) at room temperature first. The EC12 probe was later added to the reaction. Reaction samples were resolved on a native gel and fluorescent image was scanned. Lane 1 (P): EC12 probe; lane 2 (P/C): EC12 probe mixed with Cry1Ab; lanes 3–11: Cry1Ab was incubated with 4-, 8- and 16- fold of unlabeled EC1236C/44W (lanes 3–5), EC12WT (lanes 6–8), EC1244W (lanes 9–11) and BSA at 16- and 48-fold (lanes 12 and 13) followed by the addition of the EC12 probe. Arrows indicate unbound EC12 probe and Cry1Ab/EC12 complex.