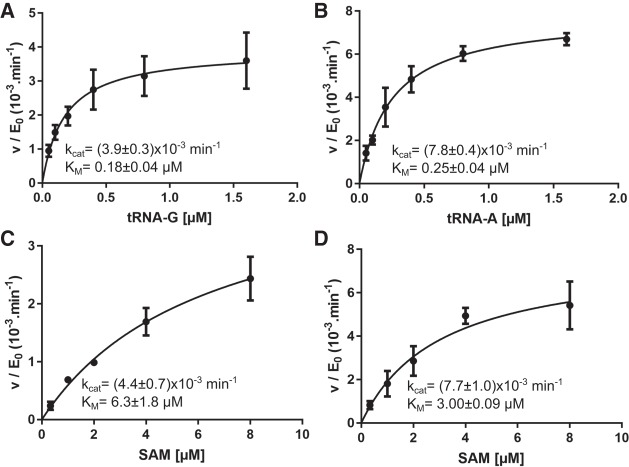
FIGURE 1.
Steady-state (Michaelis–Menten) kinetics of the methyltransferase reaction catalyzed by wild-type TkTrm10. Curves are shown using either SAM at a fixed concentration of 20 µM and varying concentrations of tRNA-G (A) or tRNA-A (B), or using SAM as a variable substrate at a fixed concentration (4 µM) of tRNA-G (C) or tRNA-A (D). Every data point is the average (±SEM) of three independent measurements. The kcat and KM values (±SE) resulting from fitting on the Michaelis–Menten equation are given in the insets.