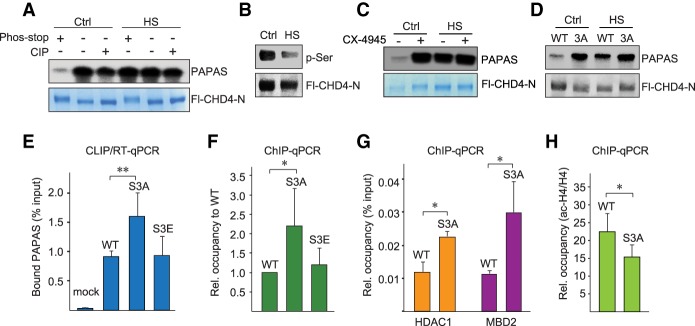
Figure 2.
Heat-induced dephosphorylation enhances RNA binding of CHD4. (A) Pull-down assays showing binding of radiolabeled PAPAS to immobilized Fl-CHD4-N from untreated (Ctrl) or heat-shocked (HS) HEK293T cells. Where indicated, Fl-CHD4-N was purified in the absence or presence of phosphatase inhibitors (phos-stop) or treated with calf intestine phosphatase (CIP). (B) Western blot with anti-phospho-serine antibody comparing phosphorylation of CHD4-N in control and heat-shocked HEK293T cells. Expression of Fl-CHD4 was monitored with anti-Flag antibody. (C) Pull-down assay showing binding of radiolabeled PAPAS to immobilized Fl-CHD4-N from control and heat-shocked HEK293T cells that were pretreated for 12 h with 5 µM CX-4945. (D) Pull-down assay showing binding of radiolabeled PAPAS to immobilized wild-type Fl-CHD4-N and mutant Fl-CHD4-N/S3A from untreated or heat-shocked HEK293T cells. (E) CLIP/RT-qPCR comparing binding of PAPAS to Flag-tagged wild-type CHD4, CHD4/S3A, and CHD4/S3E in HEK293T cells. Data were normalized to input. n = 3. (F) ChIP-qPCR assays monitoring binding of wild-type CHD4 and mutants CHD4/S3A and CHD4/S3E to the rDNA promoter. Data were normalized to wild-type CHD4. n = 3. (G) ChIP-qPCR monitoring the occupancy of HDAC1 and MBD2 at the rDNA promoter. n = 3. (H) ChIP-qPCR monitoring acetylated histone H4 at the rDNA promoter. n = 3. Data were normalized to bound histone H4. Error bars represent mean values ± SD.