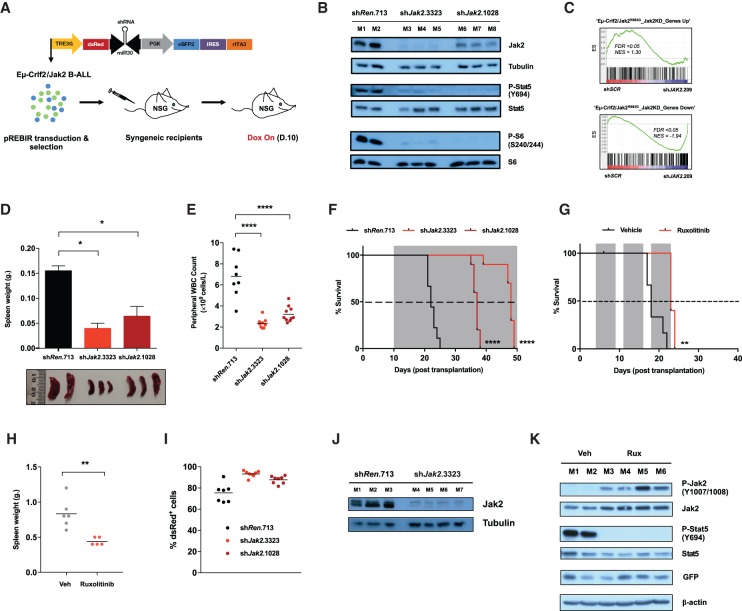
Figure 3.
Eµ-Crlf2/Jak2 mutant B-ALLs persist despite sustained Jak2 inhibition or depletion in vivo. (A) Schematic representation of the in vivo application of Dox-inducible (Tet-on) pREBIR system. Established Eµ-Crlf2/Jak2 mutant B-ALLs were freshly isolated, transduced with pREBIR harboring shRNAs targeting Jak2 (shJak2.3323 or shJak2.1028) or shRenilla.713, FACS-sorted for transduced populations (eBFP2+), and retransplanted into NSG recipient mice. shRNAs were transiently induced by Dox administration in vivo. (B) Cohorts of mice were transplanted with Eµ-Crlf2/Jak2R683G-pREBIR-shRenilla.713 (mouse 1 [M1] and mouse 2 [M2]; n = 2), Eµ-Crlf2/Jak2R683G- pREBIR-shJak2.3323 (M3–M5; n = 3), or Eµ-Crlf2/Jak2R683G-pREBIR-shJak2.1028 (M6–M8; n = 3) cells and treated with Dox for 48 h at 10 d after engraftment prior to being sacrificed. Immunoblotting was performed for the indicated targets using whole-cell lysates from dsRed+/eBFP2+ splenocytes. Tubulin served as a loading control. (C) GSEA of JAK2 knockdown human MHH-CALL4 cells at day 5 after transduction (MHH-CALL4-pLMS-shSCR [shSCR] relative to MHH-CALL4-pLMS-shJAK2.209 cells [shJAK2.209]) demonstrates significant enrichment of gene sets induced (“Eµ-Crlf2/Jak2R683G_Jak2KD_Genes Down”) and repressed (“Eµ-Crlf2/Jak2R683G_Jak2KD_Genes Up”) in Jak2 knockdown mouse Eµ-Crlf2/Jak2R683G B-ALL cells (Eµ-Crlf2/Jak2R683G-pREBIR-shJak2.3323 relative to Eµ-Crlf2/Jak2R683G-pREBIR-shRenilla.713; 48 h after shRNA induction in vivo). (D) Cohorts of recipient mice were transplanted with Eµ-Crlf2/Jak2R683G-pREBIR-shRenilla.713 (n = 2), Eµ-Crlf2/Jak2R683G-pREBIR-shJak2.3323 (n = 3), or Eµ-Crlf2/Jak2R683G-pREBIR-shJak2.1028 (n = 3) cells for 10 d and treated with Dox for 48 h prior to being sacrificed and analyzed for spleen weight. Error bars represent SEM. n = 2. A photograph of spleens from mice in each treatment arm is shown. The ruler scale is in millimeters. (E) Peripheral blood from recipient mice of Eµ-Crlf2/Jak2R683G-pREBIR-shRenilla.713 (n = 9), Eµ-Crlf2/Jak2R683G–pREBIR-shJak2.3323 (n = 10), or Eµ-Crlf2/Jak2R683G–pREBIR-shJak2.1028 (n = 10) cells at week 3 after injection was analyzed for total peripheral WBC count. All mice were treated with Dox from day 10 after tumor transplantation. (F) Kaplan-Meier survival curve of NSG recipient mice treated as in D. Gray shading indicates the period of Dox administration. (G) Kaplan-Meier survival curve of recipient mice of Eµ-Crlf2/Jak2R683G B-ALL cells treated with vehicle (0.5% methyl cellulose; n = 6) or 90 mg/kg ruxolitinib twice per day (n = 6) from day 3 after injection by oral gavage. Gray shading indicates the period of drug treatment. (H) At terminal disease, mice from G were autopsied, and splenic burden was assessed by spleen weight. (I) Quantification of dsRed+/eBFP2+ proportions in GFP+ splenocytes collected from moribund bound Dox-treated recipient mice from F at terminal disease. (J) Western blot analysis was performed for total Jak2 using lysates isolated from dsRed+/eBFP2+ splenocytes collected from individual Dox-treated moribund recipients of Eµ-Crlf2/Jak2R683G-pREBIR-shRen.713 (M1–M3; n = 3) and Eµ-Crlf2/Jak2R683G-pREBIR-shJak2.3323 (M4–M7; n = 4) cells from F at terminal disease. Tubulin served as a loading control. (K) Immunoblotting was performed against the indicated targets using lysates isolated from splenocytes collected from individual vehicle (M1–M2; n = 2) or ruxolitinib-treated (M3–M6; n = 4) moribund recipients of Eµ-Crlf2/Jak2R683G B-ALLs at terminal disease. β-Actin served as a loading control. (*) P < 0.05; (**) P < 0.01; (****) P < 0.0001.